Activation of the AP-1 transcription factor by inflammatory cytokines of the TNF family
- PMID: 10440223
- PMCID: PMC6174675
Activation of the AP-1 transcription factor by inflammatory cytokines of the TNF family
Abstract
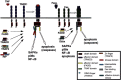
Inflammatory cytokines of the tumor necrosis factor (TNF) family mediate a large variety of cellular and organismal inflammatory responses and are important to the pathogenesis of a number of important disease states including arthritis, septic shock, inflammatory bowel disease, and, possibly, type II diabetes. Many of the responses to these cytokines require de novo gene expression mediated by the activator protein-1 (AP-1) heterodimeric transcription factor. This review will discuss what is known of how cytokines of the TNF family, acting at the cell surface, recruit two mitogen-activated protein kinase (MAPK) subfamilies, the stress-activated protein kinases (SAPKs, also called JNKs) and the p38s, to transduce signals to AP-1.
Figures




Similar articles
-
Tumor necrosis factor signaling to stress-activated protein kinase (SAPK)/Jun NH2-terminal kinase (JNK) and p38. Germinal center kinase couples TRAF2 to mitogen-activated protein kinase/ERK kinase kinase 1 and SAPK while receptor interacting protein associates with a mitogen-activated protein kinase kinase kinase upstream of MKK6 and p38.J Biol Chem. 1998 Aug 28;273(35):22681-92. doi: 10.1074/jbc.273.35.22681. J Biol Chem. 1998. PMID: 9712898
-
Calcium/calmodulin-dependent kinase II is required for platelet-activating factor priming.Shock. 2005 Feb;23(2):99-106. doi: 10.1097/01.shk.0000148075.19190.db. Shock. 2005. PMID: 15665723
-
A Mycoplasma fermentans-derived synthetic lipopeptide induces AP-1 and NF-kappaB activity and cytokine secretion in macrophages via the activation of mitogen-activated protein kinase pathways.J Biol Chem. 1998 Dec 18;273(51):34391-8. doi: 10.1074/jbc.273.51.34391. J Biol Chem. 1998. PMID: 9852105
-
Signal transduction by tumor necrosis factor and its relatives.Trends Cell Biol. 2001 Sep;11(9):372-7. doi: 10.1016/s0962-8924(01)02064-5. Trends Cell Biol. 2001. PMID: 11514191 Review.
-
TNF blockade: an inflammatory issue.Ernst Schering Res Found Workshop. 2006;(56):161-86. doi: 10.1007/3-540-37673-9_10. Ernst Schering Res Found Workshop. 2006. PMID: 16331857 Review.
Cited by
-
New Benzofuran N-Acylhydrazone Reduces Cardiovascular Dysfunction in Obese Rats by Blocking TNF-Alpha Synthesis.Drug Des Devel Ther. 2020 Aug 17;14:3337-3350. doi: 10.2147/DDDT.S258459. eCollection 2020. Drug Des Devel Ther. 2020. PMID: 32884238 Free PMC article.
-
Gene regulation of CYP4F11 in human keratinocyte HaCaT cells.Drug Metab Dispos. 2010 Jan;38(1):100-7. doi: 10.1124/dmd.109.029025. Drug Metab Dispos. 2010. PMID: 19812349 Free PMC article.
-
Gomisin N Decreases Inflammatory Cytokine Production in Human Periodontal Ligament Cells.Inflammation. 2017 Apr;40(2):360-365. doi: 10.1007/s10753-016-0482-4. Inflammation. 2017. PMID: 27896541
-
Mapping disease regulatory circuits at cell-type resolution from single-cell multiomics data.Nat Comput Sci. 2023 Jul;3(7):644-657. doi: 10.1038/s43588-023-00476-5. Epub 2023 Jul 25. Nat Comput Sci. 2023. PMID: 37974651 Free PMC article.
-
Akt phosphorylates and negatively regulates apoptosis signal-regulating kinase 1.Mol Cell Biol. 2001 Feb;21(3):893-901. doi: 10.1128/MCB.21.3.893-901.2001. Mol Cell Biol. 2001. PMID: 11154276 Free PMC article.
References
-
- Akiba H.; Nakano H.; Nishinaka S.; Shindo M.; Kobata T.; Atsuta M.; Morimoto C.; Ware C. F.; Malinin N.; Wallach D.; Yagita H.; Okumura K. CD27, a member of the tumor necrosis factor receptor super-family, activates NF-κB and stress-activated protein kinase/c-Jun N-terminal kinase via TRAF2, TRAF5 and NF-κB-inducing kinase. J. Biol. Chem. 273: 13353–13358; 1998. - PubMed
-
- Arch R. H.; Gedrich R. W.; Thompson C. B. Tumor necrosis factor receptor-associated factors (TRAFs)—a family of adapter proteins that regulates life and death. Genes Dev. 12:2821–2830; 1998. - PubMed
-
- Avruch J.; Zhang X.-f.; Kyriakis J. M. Raf meets Ras: Completing the framework of a signal transduction pathway. Trends Biochem. Sci. 19:279–283; 1994. - PubMed
-
- Blank J. L.; Gerwins P.; Elliot E. M.; Sather S.; Johnson G. L. Molecular cloning of mitogen activated protein/ERK kinase kinases (MEKK) 2 and 3. J. Biol. Chem. 271:5361–5368; 1996. - PubMed
-
- Chang H. Y.; Nishitoh H.; Yang X.; Ichijo H.; Baltimore D. Activation of apoptosis signal-regulating kinase 1 (ASK1) by the adapter protein Daxx. Science 281:1860–1863; 1998. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
