Envelope-dependent restriction of human immunodeficiency virus type 1 spreading in CD4(+) T lymphocytes: R5 but not X4 viruses replicate in the absence of T-cell receptor restimulation
- PMID: 10438841
- PMCID: PMC104278
- DOI: 10.1128/JVI.73.9.7515-7523.1999
Envelope-dependent restriction of human immunodeficiency virus type 1 spreading in CD4(+) T lymphocytes: R5 but not X4 viruses replicate in the absence of T-cell receptor restimulation
Abstract
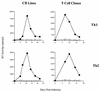
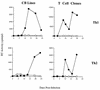
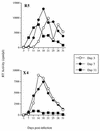
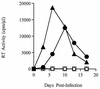
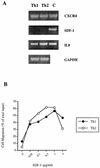
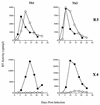
The human immunodeficiency virus (HIV) replicates in activated CD4(+) T lymphocytes. However, only CD4(+) Th2 and Th0, but not Th1, CD4(+) T-cell clones have been reported to efficiently support HIV-1 replication. This dichotomous pattern was further investigated in the present study in Th1, Th2, or Th0 cell lines derived from umbilical human cord blood and in T-cell clones obtained from the peripheral blood mononuclear cells (PBMC) of healthy adults. Both primary and laboratory-adapted HIV-1 strains with CCR5 as the exclusive entry coreceptor (R5 viruses) efficiently replicated in Th1, Th2, and Th0 cells. In sharp contrast, CXCR4-dependent (X4) viruses poorly replicated in both polarized and unpolarized CD4(+) T cells, including adults' PBMC infected several days after mitogenic stimulation. Unlike the X4 HIV-1(NL4-3), a chimera in which the env gene had been replaced with that of the R5 HIV-1(NL(AD8)), efficiently replicated in both Th1 and Th2 cells. This X4-dependent restriction of HIV replication was not explained by either the absence of functional CXCR4 on the cell surface or by the inefficient viral entry and reverse transcription. T-cell receptor stimulation by anti-CD3 monoclonal antibodies fully rescued X4 HIV-1 replication in both Th1 and Th2 cells, whereas it did not alter the extent and kinetics of R5 HIV-1 spreading. Thus, R5 HIVs show a replicative advantage in comparison to X4 viruses in their ability to efficiently propagate among suboptimally activated T lymphocytes, regardless of their polarized or unpolarized functional profiles. This observation may help to explain the absolute predominance of R5 HIVs over X4 viruses observed after viral transmission and during early-stage disease.
Figures


Similar articles
-
Inhibition of CD3/CD28-mediated activation of the MEK/ERK signaling pathway represses replication of X4 but not R5 human immunodeficiency virus type 1 in peripheral blood CD4(+) T lymphocytes.J Virol. 2000 Mar;74(6):2558-66. doi: 10.1128/jvi.74.6.2558-2566.2000. J Virol. 2000. PMID: 10684270 Free PMC article.
-
Restricted replication of primary HIV-1 isolates using both CCR5 and CXCR4 in Th2 but not in Th1 CD4(+) T cells.J Leukoc Biol. 2002 Nov;72(5):913-20. J Leukoc Biol. 2002. PMID: 12429712
-
CCR5 and CXCR4 expression correlated with X4 and R5 HIV-1 infection yet not sustained replication in Th1 and Th2 cells.AIDS. 2001 Oct 19;15(15):1941-9. doi: 10.1097/00002030-200110190-00005. AIDS. 2001. PMID: 11600821
-
HIV-1 replication in CD4+ T cell lines: the effects of adaptation on co-receptor use, tropism, and accessory gene function.J Leukoc Biol. 2000 Sep;68(3):331-7. J Leukoc Biol. 2000. PMID: 10985248 Review.
-
Relationships Between HIV-Mediated Chemokine Coreceptor Signaling, Cofilin Hyperactivation, Viral Tropism Switch and HIV-Mediated CD4 Depletion.Curr HIV Res. 2019;17(6):388-396. doi: 10.2174/1570162X17666191106112018. Curr HIV Res. 2019. PMID: 31702526 Review.
Cited by
-
Inhibition of CD3/CD28-mediated activation of the MEK/ERK signaling pathway represses replication of X4 but not R5 human immunodeficiency virus type 1 in peripheral blood CD4(+) T lymphocytes.J Virol. 2000 Mar;74(6):2558-66. doi: 10.1128/jvi.74.6.2558-2566.2000. J Virol. 2000. PMID: 10684270 Free PMC article.
-
Cell surface CCR5 density determines the postentry efficiency of R5 HIV-1 infection.Proc Natl Acad Sci U S A. 2002 Nov 26;99(24):15590-5. doi: 10.1073/pnas.242134499. Epub 2002 Nov 14. Proc Natl Acad Sci U S A. 2002. PMID: 12434015 Free PMC article.
-
Naive CD4 T cells inhibit CD28-costimulated R5 HIV replication in memory CD4 T cells.Proc Natl Acad Sci U S A. 2001 Sep 25;98(20):11644-9. doi: 10.1073/pnas.211205098. Epub 2001 Sep 18. Proc Natl Acad Sci U S A. 2001. PMID: 11562498 Free PMC article.
-
Selective inhibition of HIV replication in primary macrophages but not T lymphocytes by macrophage-derived chemokine.Proc Natl Acad Sci U S A. 2000 Aug 1;97(16):9162-7. doi: 10.1073/pnas.160359197. Proc Natl Acad Sci U S A. 2000. PMID: 10908681 Free PMC article.
-
Rational design of HIV vaccines and microbicides: report of the EUROPRISE network annual conference 2010.J Transl Med. 2011 Apr 12;9:40. doi: 10.1186/1479-5876-9-40. J Transl Med. 2011. PMID: 21486446 Free PMC article.
References
-
- Aiuti A, Turchetto L, Cota M, Cipponi A, Brambilla A, Arcelloni C, Paroni R, Vicenzi E, Bordignon C, Poli G. Human CD34+ cells express CXCR4 and its ligand stromal cell derived factor-1. Implications for infection by T-cell tropic human immunodeficiency virus. Blood. 1999;94:62–73. - PubMed
-
- Berger E A, Doms R W, Fenyo E M, Korber B T, Littman D R, Moore J P, Sattentau Q J, Schuitemaker H, Sodroski J, Weiss R A. A new classification for HIV-1. Nature. 1998;391:240. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

