Relaxed negative selection in germinal centers and impaired affinity maturation in bcl-xL transgenic mice
- PMID: 10430628
- PMCID: PMC2195593
- DOI: 10.1084/jem.190.3.399
Relaxed negative selection in germinal centers and impaired affinity maturation in bcl-xL transgenic mice
Abstract
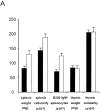
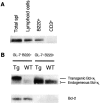
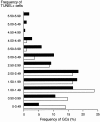
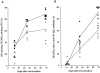
The role of apoptosis in affinity maturation was investigated by determining the affinity of (4-hydroxy-3-nitrophenyl)acetyl (NP)-specific antibody-forming cells (AFCs) and serum antibody in transgenic mice that overexpress a suppressor of apoptosis, Bcl-xL, in the B cell compartment. Although transgenic animals briefly expressed higher numbers of splenic AFCs after immunization, the bcl-xL transgene did not increase the number or size of germinal centers (GCs), alter the levels of serum antibody, or change the frequency of NP-specific, long-lived AFCs. Nonetheless, the bcl-xL transgene product, in addition to endogenous Bcl-xL, reduced apoptosis in GC B cells and resulted in the expansion of B lymphocytes bearing VDJ rearrangements that are usually rare in primary anti-NP responses. Long-lived AFCs bearing these noncanonical rearrangements were frequent in the bone marrow and secreted immunoglobulin G(1) antibodies with low affinity for NP. The abundance of noncanonical cells lowered the average affinity of long-lived AFCs and serum antibody, demonstrating that Bcl-xL and apoptosis influence clonal selection/maintenance for affinity maturation.
Figures


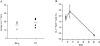
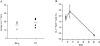
Similar articles
-
bcl-2 transgene expression inhibits apoptosis in the germinal center and reveals differences in the selection of memory B cells and bone marrow antibody-forming cells.J Exp Med. 2000 Feb 7;191(3):475-84. doi: 10.1084/jem.191.3.475. J Exp Med. 2000. PMID: 10662793 Free PMC article.
-
In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. V. Affinity maturation develops in two stages of clonal selection.J Exp Med. 1998 Mar 16;187(6):885-95. doi: 10.1084/jem.187.6.885. J Exp Med. 1998. PMID: 9500791 Free PMC article.
-
BH3-only protein Noxa regulates apoptosis in activated B cells and controls high-affinity antibody formation.Blood. 2012 Feb 9;119(6):1440-9. doi: 10.1182/blood-2011-09-378877. Epub 2011 Dec 5. Blood. 2012. PMID: 22144184
-
B lymphocyte physiology: the beginning and the end.Ciba Found Symp. 1997;204:220-30; discussion 230-1. doi: 10.1002/9780470515280.ch15. Ciba Found Symp. 1997. PMID: 9107424 Review.
-
Germinal-center-derived B-cell memory.Adv Exp Med Biol. 2007;590:139-48. doi: 10.1007/978-0-387-34814-8_10. Adv Exp Med Biol. 2007. PMID: 17191383 Review. No abstract available.
Cited by
-
Taking advantage: high-affinity B cells in the germinal center have lower death rates, but similar rates of division, compared to low-affinity cells.J Immunol. 2009 Dec 1;183(11):7314-25. doi: 10.4049/jimmunol.0902452. Epub 2009 Nov 16. J Immunol. 2009. PMID: 19917681 Free PMC article.
-
Novel targeted deregulation of c-Myc cooperates with Bcl-X(L) to cause plasma cell neoplasms in mice.J Clin Invest. 2004 Jun;113(12):1763-73. doi: 10.1172/JCI20369. J Clin Invest. 2004. PMID: 15199411 Free PMC article.
-
Regulation of germinal center responses and B-cell memory by the chromatin modifier MOZ.Proc Natl Acad Sci U S A. 2014 Jul 1;111(26):9585-90. doi: 10.1073/pnas.1402485111. Epub 2014 Jun 16. Proc Natl Acad Sci U S A. 2014. PMID: 24979783 Free PMC article.
-
CD80 expression on B cells regulates murine T follicular helper development, germinal center B cell survival, and plasma cell generation.J Immunol. 2012 May 1;188(9):4217-25. doi: 10.4049/jimmunol.1102885. Epub 2012 Mar 26. J Immunol. 2012. PMID: 22450810 Free PMC article.
-
Attenuation of apoptosis underlies B lymphocyte stimulator enhancement of humoral immune response.J Exp Med. 2000 Oct 2;192(7):953-64. doi: 10.1084/jem.192.7.953. J Exp Med. 2000. PMID: 11015437 Free PMC article.
References
-
- Eisen H.N., Siskind G.W. Variations in affinities of antibodies during the immune response. Biochemistry. 1964;3:996–1008. - PubMed
-
- Siskind G.W., Benacerraf B. Cell selection by antigen in the immune response. Adv. Immunol. 1969;10:1–50. - PubMed
-
- Griffiths G.M., Berek C., Kaartinen M., Milstein C. Somatic mutation and the maturation of the immune response. Nature. 1984;312:271–275. - PubMed
-
- French D.L., R.Laskov, Scharff M.D. The role of somatic hypermutation in the generation of antibody diversity. Science. 1989;244:1152–1157. - PubMed
-
- Nossal G.J.V., Ada G.L., Austin C.M. Antigens in immunity. IV. Cellular localization of 125I and 131I labeled flagella in lymph nodes. Aust. J. Exp. Biol. Med. Sci. 1964;42:311–330. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

