The Saccharomyces cerevisiae weak-acid-inducible ABC transporter Pdr12 transports fluorescein and preservative anions from the cytosol by an energy-dependent mechanism
- PMID: 10419965
- PMCID: PMC103598
- DOI: 10.1128/JB.181.15.4644-4652.1999
The Saccharomyces cerevisiae weak-acid-inducible ABC transporter Pdr12 transports fluorescein and preservative anions from the cytosol by an energy-dependent mechanism
Abstract
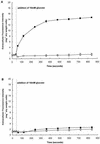
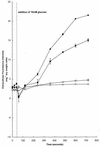
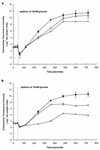
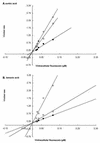
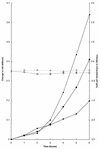
Growth of Saccharomyces cerevisiae in the presence of the weak-acid preservative sorbic acid results in the induction of the ATP-binding cassette (ABC) transporter Pdr12 in the plasma membrane (P. Piper, Y. Mahe, S. Thompson, R. Pandjaitan, C. Holyoak, R. Egner, M. Muhlbauer, P. Coote, and K. Kuchler, EMBO J. 17:4257-4265, 1998). Pdr12 appears to mediate resistance to water-soluble, monocarboxylic acids with chain lengths of from C(1) to C(7). Exposure to acids with aliphatic chain lengths greater than C(7) resulted in no observable sensitivity of Deltapdr12 mutant cells compared to the parent. Parent and Deltapdr12 mutant cells were grown in the presence of sorbic acid and subsequently loaded with fluorescein. Upon addition of an energy source in the form of glucose, parent cells immediately effluxed fluorescein from the cytosol into the surrounding medium. In contrast, under the same conditions, cells of the Deltapdr12 mutant were unable to efflux any of the dye. When both parent and Deltapdr12 mutant cells were grown without sorbic acid and subsequently loaded with fluorescein, upon the addition of glucose no efflux of fluorescein was detected from either strain. Thus, we have shown that Pdr12 catalyzes the energy-dependent extrusion of fluorescein from the cytosol. Lineweaver-Burk analysis revealed that sorbic and benzoic acids competitively inhibited ATP-dependent fluorescein efflux. Thus, these data provide strong evidence that sorbate and benzoate anions compete with fluorescein for a putative monocarboxylate binding site on the Pdr12 transporter.
Figures
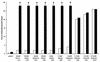


Similar articles
-
The pdr12 ABC transporter is required for the development of weak organic acid resistance in yeast.EMBO J. 1998 Aug 3;17(15):4257-65. doi: 10.1093/emboj/17.15.4257. EMBO J. 1998. PMID: 9687494 Free PMC article.
-
Loss of Cmk1 Ca(2+)-calmodulin-dependent protein kinase in yeast results in constitutive weak organic acid resistance, associated with a post-transcriptional activation of the Pdr12 ATP-binding cassette transporter.Mol Microbiol. 2000 Aug;37(3):595-605. doi: 10.1046/j.1365-2958.2000.02017.x. Mol Microbiol. 2000. PMID: 10931353
-
The diverse role of Pdr12 in resistance to weak organic acids.Yeast. 2014 Jun;31(6):219-32. doi: 10.1002/yea.3011. Epub 2014 May 6. Yeast. 2014. PMID: 24691985
-
Carboxylic Acids Plasma Membrane Transporters in Saccharomyces cerevisiae.Adv Exp Med Biol. 2016;892:229-251. doi: 10.1007/978-3-319-25304-6_9. Adv Exp Med Biol. 2016. PMID: 26721276 Review.
-
Novel stress responses facilitate Saccharomyces cerevisiae growth in the presence of the monocarboxylate preservatives.Yeast. 2008 Mar;25(3):169-77. doi: 10.1002/yea.1576. Yeast. 2008. PMID: 18240334 Review.
Cited by
-
Quantitative analysis of the modes of growth inhibition by weak organic acids in Saccharomyces cerevisiae.Appl Environ Microbiol. 2012 Dec;78(23):8377-87. doi: 10.1128/AEM.02126-12. Epub 2012 Sep 21. Appl Environ Microbiol. 2012. PMID: 23001666 Free PMC article.
-
Comparative transcriptome assembly and genome-guided profiling for Brettanomyces bruxellensis LAMAP2480 during p-coumaric acid stress.Sci Rep. 2016 Sep 28;6:34304. doi: 10.1038/srep34304. Sci Rep. 2016. PMID: 27678167 Free PMC article.
-
Functional expression of the lactate permease Jen1p of Saccharomyces cerevisiae in Pichia pastoris.Biochem J. 2003 Dec 15;376(Pt 3):781-7. doi: 10.1042/BJ20031180. Biochem J. 2003. PMID: 12962538 Free PMC article.
-
Hormetic concentrations of hydrogen peroxide but not ethanol induce cross-adaptation to different stresses in budding yeast.Int J Microbiol. 2014;2014:485792. doi: 10.1155/2014/485792. Epub 2014 Jan 14. Int J Microbiol. 2014. PMID: 24669223 Free PMC article.
-
Weak acid and alkali stress regulate phosphatidylinositol bisphosphate synthesis in Saccharomyces cerevisiae.Biochem J. 2006 Apr 1;395(1):73-80. doi: 10.1042/BJ20051765. Biochem J. 2006. PMID: 16316315 Free PMC article.
References
-
- Balzi E, Goffeau A. Genetics and biochemistry of yeast multidrug resistance. Biochim Biophys Acta. 1994;1187:152–162. - PubMed
-
- Bissinger P H, Kuchler K. Molecular cloning and expression of the Saccharomyces cerevisiae STS1 gene product. A yeast ABC transporter conferring mycotoxin resistance. J Biol Chem. 1994;269:4180–4186. - PubMed
-
- Bolhuis H, van Veen H W, Poolman B, Driessen A J M, Konings W N. Mechanisms of multidrug transporters. FEMS Microbiol Rev. 1997;21:55–84. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

