A systematic study of low-resolution recognition in protein--protein complexes
- PMID: 10411900
- PMCID: PMC17541
- DOI: 10.1073/pnas.96.15.8477
A systematic study of low-resolution recognition in protein--protein complexes
Abstract
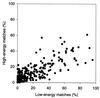
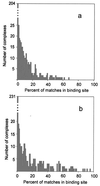
A comprehensive nonredundant database of 475 cocrystallized protein-protein complexes was used to study low-resolution recognition, which was reported in earlier docking experiments with a small number of proteins. The docking program GRAMM was used to delete the atom-size structural details and systematically dock the resulting molecular images. The results reveal the existence of the low-resolution recognition in 52% of all complexes in the database and in 76% of the 113 complexes with an interface area >4,000 A(2). Limitations of the docking and analysis tools used in this study suggest that the actual number of complexes with the low-resolution recognition is higher. However, the results already prove the existence of the low-resolution recognition on a broad scale.
Figures


Similar articles
-
Docking of protein models.Protein Sci. 2002 Aug;11(8):1888-96. doi: 10.1110/ps.4730102. Protein Sci. 2002. PMID: 12142443 Free PMC article.
-
Shape complementarity of protein-protein complexes at multiple resolutions.Proteins. 2009 May 1;75(2):453-67. doi: 10.1002/prot.22256. Proteins. 2009. PMID: 18837463 Free PMC article.
-
Q-Dock: Low-resolution flexible ligand docking with pocket-specific threading restraints.J Comput Chem. 2008 Jul 30;29(10):1574-88. doi: 10.1002/jcc.20917. J Comput Chem. 2008. PMID: 18293308 Free PMC article.
-
Orthogonal multipolar interactions in structural chemistry and biology.Angew Chem Int Ed Engl. 2005 Mar 11;44(12):1788-805. doi: 10.1002/anie.200462213. Angew Chem Int Ed Engl. 2005. PMID: 15706577 Review.
-
Protein flexibility and ligand recognition: challenges for molecular modeling.Curr Top Med Chem. 2011;11(2):192-210. doi: 10.2174/156802611794863571. Curr Top Med Chem. 2011. PMID: 20939788 Review.
Cited by
-
Computational simulation of the docking of Prochlorothrix hollandica plastocyanin to potosystem I: modeling the electron transfer complex.Biophys J. 2002 Jun;82(6):3305-13. doi: 10.1016/s0006-3495(02)75671-3. Biophys J. 2002. PMID: 12023253 Free PMC article.
-
Rotation of artificial rotor axles in rotary molecular motors.Proc Natl Acad Sci U S A. 2016 Oct 4;113(40):11214-11219. doi: 10.1073/pnas.1605640113. Epub 2016 Sep 19. Proc Natl Acad Sci U S A. 2016. PMID: 27647891 Free PMC article.
-
Docking of protein models.Protein Sci. 2002 Aug;11(8):1888-96. doi: 10.1110/ps.4730102. Protein Sci. 2002. PMID: 12142443 Free PMC article.
-
Interaction between heptad repeat 1 and 2 regions in spike protein of SARS-associated coronavirus: implications for virus fusogenic mechanism and identification of fusion inhibitors.Lancet. 2004 Mar 20;363(9413):938-47. doi: 10.1016/S0140-6736(04)15788-7. Lancet. 2004. PMID: 15043961 Free PMC article.
-
Protein-protein docking with multiple residue conformations and residue substitutions.Protein Sci. 2002 Jun;11(6):1393-408. doi: 10.1110/ps.2830102. Protein Sci. 2002. PMID: 12021438 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

