Initiation of genomic plus-strand RNA synthesis from DNA and RNA templates by a viral RNA-dependent RNA polymerase
- PMID: 10400734
- PMCID: PMC112721
- DOI: 10.1128/JVI.73.8.6415-6423.1999
Initiation of genomic plus-strand RNA synthesis from DNA and RNA templates by a viral RNA-dependent RNA polymerase
Abstract
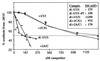
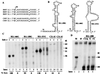
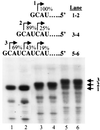
In contrast to the synthesis of minus-strand genomic and plus-strand subgenomic RNAs, the requirements for brome mosaic virus (BMV) genomic plus-strand RNA synthesis in vitro have not been previously reported. Therefore, little is known about the biochemical requirements for directing genomic plus-strand synthesis. Using DNA templates to characterize the requirements for RNA-dependent RNA polymerase template recognition, we found that initiation from the 3' end of a template requires one nucleotide 3' of the initiation nucleotide. The addition of a nontemplated nucleotide at the 3' end of minus-strand BMV RNAs led to initiation of genomic plus-strand RNA in vitro. Genomic plus-strand initiation was specific since cucumber mosaic virus minus-strand RNA templates were unable to direct efficient synthesis under the same conditions. In addition, mutational analysis of the minus-strand template revealed that the -1 nontemplated nucleotide, along with the +1 cytidylate and +2 adenylate, is important for RNA-dependent RNA polymerase interaction. Furthermore, genomic plus-strand RNA synthesis is affected by sequences 5' of the initiation site.
Figures


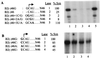
Similar articles
-
Replicase-binding sites on plus- and minus-strand brome mosaic virus RNAs and their roles in RNA replication in plant cells.J Virol. 2004 Dec;78(24):13420-9. doi: 10.1128/JVI.78.24.13420-13429.2004. J Virol. 2004. PMID: 15564452 Free PMC article.
-
Template sequence near the initiation nucleotide can modulate brome mosaic virus RNA accumulation in plant protoplasts.J Virol. 2004 Feb;78(3):1169-80. doi: 10.1128/jvi.78.3.1169-1180.2004. J Virol. 2004. PMID: 14722272 Free PMC article.
-
Recognition of the core RNA promoter for minus-strand RNA synthesis by the replicases of Brome mosaic virus and Cucumber mosaic virus.J Virol. 2000 Nov;74(22):10323-31. doi: 10.1128/jvi.74.22.10323-10331.2000. J Virol. 2000. PMID: 11044076 Free PMC article.
-
Common replication strategies emerging from the study of diverse groups of positive-strand RNA viruses.Arch Virol Suppl. 1994;9:181-94. doi: 10.1007/978-3-7091-9326-6_18. Arch Virol Suppl. 1994. PMID: 8032249 Review.
-
RNA recombination in brome mosaic virus, a model plus strand RNA virus.Acta Biochim Pol. 1998;45(4):847-68. Acta Biochim Pol. 1998. PMID: 10397334 Review.
Cited by
-
The conserved, 5' termini of RNAs 1 and 2 of Tomato aspermy virus are dispensable for infection but affect virulence.Virus Genes. 2005 Mar;30(2):181-91. doi: 10.1007/s11262-004-5626-1. Virus Genes. 2005. PMID: 15744575
-
Structural and functional analysis of the cis-acting elements required for plus-strand RNA synthesis of Bamboo mosaic virus.J Virol. 2005 Jul;79(14):9046-53. doi: 10.1128/JVI.79.14.9046-9053.2005. J Virol. 2005. PMID: 15994798 Free PMC article.
-
Non-DNA-templated addition of nucleotides to the 3' end of RNAs by the mitochondrial RNA polymerase of Physarum polycephalum.Mol Cell Biol. 2008 Sep;28(18):5795-802. doi: 10.1128/MCB.00356-08. Epub 2008 Jun 23. Mol Cell Biol. 2008. PMID: 18573885 Free PMC article.
-
Polymerization of nontemplate bases before transcription initiation at the 3' ends of templates by an RNA-dependent RNA polymerase: an activity involved in 3' end repair of viral RNAs.Proc Natl Acad Sci U S A. 2000 Nov 7;97(23):12451-6. doi: 10.1073/pnas.97.23.12451. Proc Natl Acad Sci U S A. 2000. PMID: 11070075 Free PMC article.
-
Replicase-binding sites on plus- and minus-strand brome mosaic virus RNAs and their roles in RNA replication in plant cells.J Virol. 2004 Dec;78(24):13420-9. doi: 10.1128/JVI.78.24.13420-13429.2004. J Virol. 2004. PMID: 15564452 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

