Direct involvement of ezrin/radixin/moesin (ERM)-binding membrane proteins in the organization of microvilli in collaboration with activated ERM proteins
- PMID: 10385528
- PMCID: PMC2133160
- DOI: 10.1083/jcb.145.7.1497
Direct involvement of ezrin/radixin/moesin (ERM)-binding membrane proteins in the organization of microvilli in collaboration with activated ERM proteins
Abstract
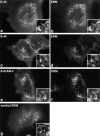
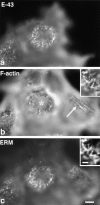
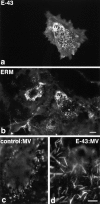
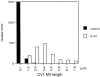
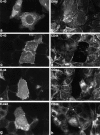
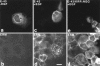
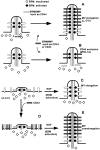
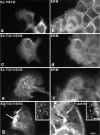
Ezrin/radixin/moesin (ERM) proteins have been thought to play a central role in the organization of cortical actin-based cytoskeletons including microvillar formation through cross-linking actin filaments and integral membrane proteins such as CD43, CD44, and ICAM-2. To examine the functions of these ERM-binding membrane proteins (ERMBMPs) in cortical morphogenesis, we overexpressed ERMBMPs (the extracellular domain of E-cadherin fused with the transmembrane/cytoplasmic domain of CD43, CD44, or ICAM-2) in various cultured cells. In cultured fibroblasts such as L and CV-1 cells, their overexpression significantly induced microvillar elongation, recruiting ERM proteins and actin filaments. When the ERM-binding domains were truncated from these molecules, their ability to induce microvillar elongation became undetectable. In contrast, in cultured epithelial cells such as MTD-1A and A431 cells, the overexpression of ERMBMPs did not elongate microvilli. However, in the presence of EGF, overexpression of ERMBMPs induced remarkable microvillar elongation in A431 cells. These results indicated that ERMBMPs function as organizing centers for cortical morphogenesis by organizing microvilli in collaboration with activated ERM proteins. Furthermore, immunodetection with a phosphorylated ERM-specific antibody and site-directed mutagenesis suggested that ERM proteins phosphorylated at their COOH-terminal threonine residue represent activated ERM proteins.
Figures



Similar articles
-
Ezrin/radixin/moesin (ERM) proteins bind to a positively charged amino acid cluster in the juxta-membrane cytoplasmic domain of CD44, CD43, and ICAM-2.J Cell Biol. 1998 Feb 23;140(4):885-95. doi: 10.1083/jcb.140.4.885. J Cell Biol. 1998. PMID: 9472040 Free PMC article.
-
Rho-dependent and -independent activation mechanisms of ezrin/radixin/moesin proteins: an essential role for polyphosphoinositides in vivo.J Cell Sci. 2002 Jun 15;115(Pt 12):2569-80. doi: 10.1242/jcs.115.12.2569. J Cell Sci. 2002. PMID: 12045227
-
Differential expression of ezrin/radixin/moesin (ERM) and ERM-associated adhesion molecules in the blastocyst and uterus suggests their functions during implantation.Biol Reprod. 2004 Mar;70(3):729-36. doi: 10.1095/biolreprod.103.022764. Epub 2003 Nov 12. Biol Reprod. 2004. PMID: 14613898
-
Cortical actin organization: lessons from ERM (ezrin/radixin/moesin) proteins.J Biol Chem. 1999 Dec 3;274(49):34507-10. doi: 10.1074/jbc.274.49.34507. J Biol Chem. 1999. PMID: 10574907 Review. No abstract available.
-
ERM proteins: from cellular architecture to cell signaling.Biol Cell. 2000 Aug;92(5):305-16. doi: 10.1016/s0248-4900(00)01078-9. Biol Cell. 2000. PMID: 11071040 Review.
Cited by
-
A member of the Plasmodium falciparum PHIST family binds to the erythrocyte cytoskeleton component band 4.1.Malar J. 2013 May 11;12:160. doi: 10.1186/1475-2875-12-160. Malar J. 2013. PMID: 23663475 Free PMC article.
-
Ezrin regulates E-cadherin-dependent adherens junction assembly through Rac1 activation.Mol Biol Cell. 2003 May;14(5):2181-91. doi: 10.1091/mbc.e02-07-0410. Epub 2003 Feb 6. Mol Biol Cell. 2003. PMID: 12802084 Free PMC article.
-
CD43 interaction with ezrin-radixin-moesin (ERM) proteins regulates T-cell trafficking and CD43 phosphorylation.Mol Biol Cell. 2011 Apr;22(7):954-63. doi: 10.1091/mbc.E10-07-0586. Epub 2011 Feb 2. Mol Biol Cell. 2011. PMID: 21289089 Free PMC article.
-
Pathogenesis of Afa/Dr diffusely adhering Escherichia coli.Clin Microbiol Rev. 2005 Apr;18(2):264-92. doi: 10.1128/CMR.18.2.264-292.2005. Clin Microbiol Rev. 2005. PMID: 15831825 Free PMC article. Review.
-
Insight into the molecular basis of pathogen abundance: group A Streptococcus inhibitor of complement inhibits bacterial adherence and internalization into human cells.Proc Natl Acad Sci U S A. 2002 May 28;99(11):7646-51. doi: 10.1073/pnas.112039899. Proc Natl Acad Sci U S A. 2002. PMID: 12032337 Free PMC article.
References
-
- Arpin M, Algrain M, Louvard D. Membrane-actin microfilament connections: an increasing diversity of players related to band 4.1. Curr Opin Cell Biol. 1994a;6:136–141. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

