Cyclic AMP-dependent protein kinase regulates pseudohyphal differentiation in Saccharomyces cerevisiae
- PMID: 10373537
- PMCID: PMC84286
- DOI: 10.1128/MCB.19.7.4874
Cyclic AMP-dependent protein kinase regulates pseudohyphal differentiation in Saccharomyces cerevisiae
Abstract
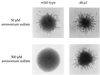
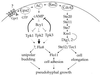
In response to nitrogen starvation, diploid cells of the yeast Saccharomyces cerevisiae differentiate to a filamentous growth form known as pseudohyphal differentiation. Filamentous growth is regulated by elements of the pheromone mitogen-activated protein (MAP) kinase cascade and a second signaling cascade involving the receptor Gpr1, the Galpha protein Gpa2, Ras2, and cyclic AMP (cAMP). We show here that the Gpr1-Gpa2-cAMP pathway signals via the cAMP-dependent protein kinase, protein kinase A (PKA), to regulate pseudohyphal differentiation. Activation of PKA by mutation of the regulatory subunit Bcy1 enhances filamentous growth. Mutation and overexpression of the PKA catalytic subunits reveal that the Tpk2 catalytic subunit activates filamentous growth, whereas the Tpk1 and Tpk3 catalytic subunits inhibit filamentous growth. The PKA pathway regulates unipolar budding and agar invasion, whereas the MAP kinase cascade regulates cell elongation and invasion. Epistasis analysis supports a model in which PKA functions downstream of the Gpr1 receptor and the Gpa2 and Ras2 G proteins. Activation of filamentous growth by PKA does not require the transcription factors Ste12 and Tec1 of the MAP kinase cascade, Phd1, or the PKA targets Msn2 and Msn4. PKA signals pseudohyphal growth, in part, by regulating Flo8-dependent expression of the cell surface flocculin Flo11. In summary, the cAMP-dependent protein kinase plays an intimate positive and negative role in regulating filamentous growth, and these findings may provide insight into the roles of PKA in mating, morphogenesis, and virulence in other yeasts and pathogenic fungi.
Figures








Similar articles
-
The G protein-coupled receptor gpr1 is a nutrient sensor that regulates pseudohyphal differentiation in Saccharomyces cerevisiae.Genetics. 2000 Feb;154(2):609-22. doi: 10.1093/genetics/154.2.609. Genetics. 2000. PMID: 10655215 Free PMC article.
-
Yeast pseudohyphal growth is regulated by GPA2, a G protein alpha homolog.EMBO J. 1997 Dec 1;16(23):7008-18. doi: 10.1093/emboj/16.23.7008. EMBO J. 1997. PMID: 9384580 Free PMC article.
-
Novel sensing mechanisms and targets for the cAMP-protein kinase A pathway in the yeast Saccharomyces cerevisiae.Mol Microbiol. 1999 Sep;33(5):904-18. doi: 10.1046/j.1365-2958.1999.01538.x. Mol Microbiol. 1999. PMID: 10476026 Review.
-
The MEP2 ammonium permease regulates pseudohyphal differentiation in Saccharomyces cerevisiae.EMBO J. 1998 Aug 10;17(5):1236-47. doi: 10.1093/emboj/17.5.1236. EMBO J. 1998. PMID: 9482721 Free PMC article.
-
Signal transduction cascades regulating pseudohyphal differentiation of Saccharomyces cerevisiae.Curr Opin Microbiol. 2000 Dec;3(6):567-72. doi: 10.1016/s1369-5274(00)00142-9. Curr Opin Microbiol. 2000. PMID: 11121775 Review.
Cited by
-
Ime1 and Ime2 are required for pseudohyphal growth of Saccharomyces cerevisiae on nonfermentable carbon sources.Mol Cell Biol. 2010 Dec;30(23):5514-30. doi: 10.1128/MCB.00390-10. Epub 2010 Sep 27. Mol Cell Biol. 2010. PMID: 20876298 Free PMC article.
-
The yeast ras/cyclic AMP pathway induces invasive growth by suppressing the cellular stress response.Mol Cell Biol. 1999 Nov;19(11):7529-38. doi: 10.1128/MCB.19.11.7529. Mol Cell Biol. 1999. PMID: 10523641 Free PMC article.
-
Messengers for morphogenesis: inositol polyphosphate signaling and yeast pseudohyphal growth.Curr Genet. 2019 Feb;65(1):119-125. doi: 10.1007/s00294-018-0874-0. Epub 2018 Aug 12. Curr Genet. 2019. PMID: 30101372 Review.
-
Glucose depletion causes haploid invasive growth in yeast.Proc Natl Acad Sci U S A. 2000 Dec 5;97(25):13619-24. doi: 10.1073/pnas.240345197. Proc Natl Acad Sci U S A. 2000. PMID: 11095711 Free PMC article.
-
Recruitment of the Swi/Snf complex by Ste12-Tec1 promotes Flo8-Mss11-mediated activation of STA1 expression.Mol Cell Biol. 2004 Nov;24(21):9542-56. doi: 10.1128/MCB.24.21.9542-9556.2004. Mol Cell Biol. 2004. PMID: 15485921 Free PMC article.
References
-
- Alspaugh J A, Perfect J R, Heitman J. Signal transduction pathways regulating differentiation and pathogenicity of Cryptococcus neoformans. Fungal Genet Biol. 1998;25:1–14. - PubMed
-
- Broek D, Samiy N, Fasano O, Fujiyama A, Tamanoi F, Northup J, Wigler M. Differential activation of yeast adenylate cyclase by wild-type and mutant ras proteins. Cell. 1985;41:763–769. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
