Replication of murine cytomegalovirus in differentiated macrophages as a determinant of viral pathogenesis
- PMID: 10364349
- PMCID: PMC112658
- DOI: 10.1128/JVI.73.7.5970-5980.1999
Replication of murine cytomegalovirus in differentiated macrophages as a determinant of viral pathogenesis
Abstract
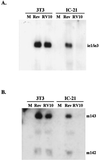
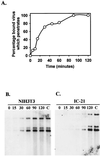
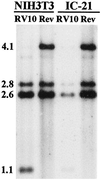
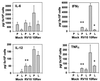
Blood monocytes or tissue macrophages play a pivotal role in the pathogenesis of murine cytomegalovirus (MCMV) infection, providing functions beneficial to both the virus and the host. In vitro and in vivo studies have indicated that differentiated macrophages support MCMV replication, are target cells for MCMV infection within tissues, and harbor latent MCMV DNA. However, this cell type presumably initiates early, antiviral immune responses as well. In addressing this paradoxical role of macrophages, we provide evidence that the proficiency of MCMV replication in macrophages positively correlates with virulence in vivo. An MCMV mutant from which the open reading frames M139, M140, and M141 had been deleted (RV10) was defective in its ability to replicate in macrophages in vitro and was highly attenuated for growth in vivo. However, depletion of splenic macrophages significantly enhanced, rather than deterred, replication of both wild-type (WT) virus and RV10 in the spleen. The ability of RV10 to replicate in intact or macrophage-depleted spleens was independent of cytokine production, as this mutant virus was a poor inducer of cytokines compared to WT virus in both intact organs and macrophage-depleted organs. Macrophages were, however, a major contributor to the production of tumor necrosis factor alpha and gamma interferon in response to WT virus infection. Thus, the data indicate that tissue macrophages serve a net protective role and may function as "filters" in protecting other highly permissive cell types from MCMV infection. The magnitude of virus replication in tissue macrophages may dictate the amount of virus accessible to the other cells. Concomitantly, infection of this cell type initiates the production of antiviral immune responses to guarantee efficient clearance of acute MCMV infection.
Figures






Similar articles
-
Products of US22 genes M140 and M141 confer efficient replication of murine cytomegalovirus in macrophages and spleen.J Virol. 2001 Jul;75(14):6292-302. doi: 10.1128/JVI.75.14.6292-6302.2001. J Virol. 2001. PMID: 11413295 Free PMC article.
-
Murine cytomegalovirus with a deletion of genes spanning HindIII-J and -I displays altered cell and tissue tropism.J Virol. 1996 Mar;70(3):1365-74. doi: 10.1128/JVI.70.3.1365-1374.1996. J Virol. 1996. PMID: 8627652 Free PMC article.
-
Role of murine cytomegalovirus US22 gene family members in replication in macrophages.J Virol. 2003 May;77(10):5557-70. doi: 10.1128/jvi.77.10.5557-5570.2003. J Virol. 2003. PMID: 12719548 Free PMC article.
-
Genetic analyses of gene function and pathogenesis of murine cytomegalovirus by transposon-mediated mutagenesis.J Clin Virol. 2002 Aug;25 Suppl 2:S111-22. doi: 10.1016/s1386-6532(02)00096-3. J Clin Virol. 2002. PMID: 12361762 Review.
-
Resisting viral infection: the gene by gene approach.Curr Opin Virol. 2011 Dec;1(6):513-8. doi: 10.1016/j.coviro.2011.10.005. Epub 2011 Nov 6. Curr Opin Virol. 2011. PMID: 22440911 Free PMC article. Review.
Cited by
-
Products of US22 genes M140 and M141 confer efficient replication of murine cytomegalovirus in macrophages and spleen.J Virol. 2001 Jul;75(14):6292-302. doi: 10.1128/JVI.75.14.6292-6302.2001. J Virol. 2001. PMID: 11413295 Free PMC article.
-
SOCS and Herpesviruses, With Emphasis on Cytomegalovirus Retinitis.Front Immunol. 2019 Apr 11;10:732. doi: 10.3389/fimmu.2019.00732. eCollection 2019. Front Immunol. 2019. PMID: 31031749 Free PMC article. Review.
-
Murine cytomegalovirus capsid assembly is dependent on US22 family gene M140 in infected macrophages.J Virol. 2009 Aug;83(15):7449-56. doi: 10.1128/JVI.00325-09. Epub 2009 May 20. J Virol. 2009. PMID: 19458005 Free PMC article.
-
Murine cytomegaloviruses m139 targets DDX3 to curtail interferon production and promote viral replication.PLoS Pathog. 2020 Oct 8;16(10):e1008546. doi: 10.1371/journal.ppat.1008546. eCollection 2020 Oct. PLoS Pathog. 2020. PMID: 33031466 Free PMC article.
-
Human cytomegalovirus transmission from the uterus to the placenta correlates with the presence of pathogenic bacteria and maternal immunity.J Virol. 2003 Dec;77(24):13301-14. doi: 10.1128/jvi.77.24.13301-13314.2003. J Virol. 2003. PMID: 14645586 Free PMC article.
References
-
- Bancroft G J, Kelly J P. Macrophage activation and innate resistance to infection in SCID mice. Immunobiology. 1994;191:424–431. - PubMed
-
- Chong K T, Mims C A. Murine cytomegalovirus particle types in relation to sources of virus and pathogenicity. J Gen Virol. 1981;57:415–419. - PubMed
-
- Compton T, Nowlin D M, Cooper N R. Initiation of human cytomegalovirus infection requires initial interaction with cell surface heparan sulfate. Virology. 1993;193:834–841. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

