Formation of virus assembly intermediate complexes in the cytoplasm by wild-type and assembly-defective mutant human immunodeficiency virus type 1 and their association with membranes
- PMID: 10364315
- PMCID: PMC112624
- DOI: 10.1128/JVI.73.7.5654-5662.1999
Formation of virus assembly intermediate complexes in the cytoplasm by wild-type and assembly-defective mutant human immunodeficiency virus type 1 and their association with membranes
Abstract
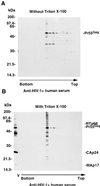
We have previously identified two distinct forms of putative viral assembly intermediate complexes, a detergent-resistant complex (DRC) and a detergent-sensitive complex (DSC), in human immunodeficiency virus type 1 (HIV-1)-infected CD4(+) T cells (Y. M. Lee and X. F. Yu, Virology 243:78-93, 1998). In the present study, the intracellular localization of these two viral assembly intermediate complexes was investigated by use of a newly developed method of subcellular fractionation. In wild-type HIV-1-infected H9 cells, the DRC fractionated with the soluble cytoplasmic fraction, whereas the DSC was associated with the membrane fraction. The DRC was also detected in the cytoplasmic fraction in H9 cells expressing HIV-1 Myr- mutant Gag. However, little of the unmyristylated Gag and Gag-Pol proteins was found in the membrane fraction. Furthermore, HIV-1 Gag proteins synthesized in vitro in a rabbit reticulocyte lysate system in the absence of exogenous lipid membrane were able to assemble into a viral Gag complex similar to that of the DRC identified in infected H9 cells. The density of the viral Gag complex was not altered by treatment with the nonionic detergent Triton X-100, suggesting a lack of association of this complex with endogenous lipid. Formation of the DRC was not significantly affected by mutations in assembly domains M and L of the Gag protein but was drastically inhibited by a mutation in the assembly I domain. Purified DRC could be disrupted by high-salt treatment, suggesting electrostatic interactions are important for stabilizing the DRC. The Gag precursor proteins in the DRC were more sensitive to trypsin digestion than those in the DSC. These findings suggest that HIV-1 Gag and Gag-Pol precursors assemble into DRC in the cytoplasm, a process which requires the protein-protein interaction domain (I) in NCp7; subsequently, the DRC is transported to the plasma membrane through a process mediated by the M domain of the matrix protein. It appears that during this process, a conformational change might occur in the DRC either before or after its association with the plasma membrane, and this change is followed by the detection of virus budding structure at the plasma membrane.
Figures






Similar articles
-
Identification and characterization of virus assembly intermediate complexes in HIV-1-infected CD4+ T cells.Virology. 1998 Mar 30;243(1):78-93. doi: 10.1006/viro.1998.9064. Virology. 1998. PMID: 9527917
-
Kinetic analysis of human immunodeficiency virus type 1 assembly reveals the presence of sequential intermediates.J Virol. 2000 Jul;74(13):5845-55. doi: 10.1128/jvi.74.13.5845-5855.2000. J Virol. 2000. PMID: 10846064 Free PMC article.
-
A bipartite membrane-binding signal in the human immunodeficiency virus type 1 matrix protein is required for the proteolytic processing of Gag precursors in a cell type-dependent manner.J Virol. 1998 Nov;72(11):9061-8. doi: 10.1128/JVI.72.11.9061-9068.1998. J Virol. 1998. PMID: 9765451 Free PMC article.
-
The Assembly of HTLV-1-How Does It Differ from HIV-1?Viruses. 2024 Sep 27;16(10):1528. doi: 10.3390/v16101528. Viruses. 2024. PMID: 39459862 Free PMC article. Review.
-
HIV-1 assembly - when virology meets biophysics.J Cell Sci. 2024 Oct 1;137(19):jcs262064. doi: 10.1242/jcs.262064. Epub 2024 Oct 15. J Cell Sci. 2024. PMID: 39404604 Review.
Cited by
-
HIV Gag-leucine zipper chimeras form ABCE1-containing intermediates and RNase-resistant immature capsids similar to those formed by wild-type HIV-1 Gag.J Virol. 2011 Jul;85(14):7419-35. doi: 10.1128/JVI.00288-11. Epub 2011 May 4. J Virol. 2011. PMID: 21543480 Free PMC article.
-
Virus entry, assembly, budding, and membrane rafts.Microbiol Mol Biol Rev. 2003 Jun;67(2):226-37, table of contents. doi: 10.1128/MMBR.67.2.226-237.2003. Microbiol Mol Biol Rev. 2003. PMID: 12794191 Free PMC article. Review.
-
Single Viruses on the Fluorescence Microscope: Imaging Molecular Mobility, Interactions and Structure Sheds New Light on Viral Replication.Viruses. 2018 May 10;10(5):250. doi: 10.3390/v10050250. Viruses. 2018. PMID: 29748498 Free PMC article. Review.
-
How HIV-1 Gag assembles in cells: Putting together pieces of the puzzle.Virus Res. 2014 Nov 26;193:89-107. doi: 10.1016/j.virusres.2014.07.001. Epub 2014 Jul 24. Virus Res. 2014. PMID: 25066606 Free PMC article. Review.
-
Analysis of human immunodeficiency virus type 1 Gag dimerization-induced assembly.J Virol. 2005 Dec;79(23):14498-506. doi: 10.1128/JVI.79.23.14498-14506.2005. J Virol. 2005. PMID: 16282449 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

