p53-Independent and -dependent requirements for E1B-55K in adenovirus type 5 replication
- PMID: 10364280
- PMCID: PMC112589
- DOI: 10.1128/JVI.73.7.5333-5344.1999
p53-Independent and -dependent requirements for E1B-55K in adenovirus type 5 replication
Abstract
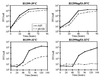
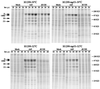
The adenovirus type 5 mutant dl1520 was engineered previously to be completely defective for E1B-55K functions. Recently, this mutant (also known as ONYX-015) has been suggested to replicate preferentially in p53(-) and some p53(+) tumor cell lines but to be attenuated in primary cultured cells (C. Heise, A. Sampson-Johannes, A. Williams, F. McCormick, D. D. F. Hoff, and D. H. Kirn, Nat. Med. 3:639-645, 1997). It has been suggested that dl1520 might be used as a "magic bullet" that could selectively lyse tumor cells without harm to normal tissues. However, we report here that dl1520 replication is independent of p53 genotype and occurs efficiently in some primary cultured human cells, indicating that the mutant virus does not possess a tumor selectivity. Although it was not the sole host range determinant, p53 function did reduce dl1520 replication when analyzed in a cell line expressing temperature-sensitive p53 (H1299-tsp53) (K. L. Fries, W. E. Miller, and N. Raab-Traub, J. Virol. 70:8653-8659, 1996). As found earlier for other E1B-55K mutants in HeLa cells (Y. Ho, R. Galos, and J. Williams, Virology 122:109-124, 1982), dl1520 replication was temperature dependent in H1299 cells. When p53 function was restored at low temperature in H1299-tsp53 cells, it imposed a modest defect in viral DNA replication and accumulation of late viral cytoplasmic mRNA. However, in both H1299 and H1299-tsp53 cells, the defect in late viral protein synthesis appeared to be much greater than could be accounted for by the modest defects in late viral mRNA levels. We therefore propose that in addition to countering p53 function and modulating viral and cellular mRNA nuclear transport, E1B-55K also stimulates late viral mRNA translation.
Figures



Similar articles
-
p53 status does not determine outcome of E1B 55-kilodalton mutant adenovirus lytic infection.J Virol. 1998 Dec;72(12):9479-90. doi: 10.1128/JVI.72.12.9479-9490.1998. J Virol. 1998. PMID: 9811681 Free PMC article.
-
Analyses of single-amino-acid substitution mutants of adenovirus type 5 E1B-55K protein.J Virol. 2001 May;75(9):4297-307. doi: 10.1128/JVI.75.9.4297-4307.2001. J Virol. 2001. PMID: 11287579 Free PMC article.
-
Late viral RNA export, rather than p53 inactivation, determines ONYX-015 tumor selectivity.Cancer Cell. 2004 Dec;6(6):611-23. doi: 10.1016/j.ccr.2004.11.012. Cancer Cell. 2004. PMID: 15607965
-
Interactions between adenovirus proteins and the p53 pathway: the development of ONYX-015.Semin Cancer Biol. 2000 Dec;10(6):453-9. doi: 10.1006/scbi.2000.0336. Semin Cancer Biol. 2000. PMID: 11170867 Review.
-
Genetically modified adenoviruses against gliomas: from bench to bedside.Neurology. 2004 Aug 10;63(3):418-26. doi: 10.1212/01.wnl.0000133302.15022.7f. Neurology. 2004. PMID: 15304571 Review.
Cited by
-
Concepts in Oncolytic Adenovirus Therapy.Int J Mol Sci. 2021 Sep 29;22(19):10522. doi: 10.3390/ijms221910522. Int J Mol Sci. 2021. PMID: 34638863 Free PMC article. Review.
-
The human adenovirus 5 L4 promoter is activated by cellular stress response protein p53.J Virol. 2013 Nov;87(21):11617-25. doi: 10.1128/JVI.01924-13. Epub 2013 Aug 21. J Virol. 2013. PMID: 23966406 Free PMC article.
-
Role of the RNA recognition motif of the E1B 55 kDa protein in the adenovirus type 5 infectious cycle.Virology. 2011 Aug 15;417(1):9-17. doi: 10.1016/j.virol.2011.04.014. Epub 2011 May 24. Virology. 2011. PMID: 21605885 Free PMC article.
-
Analysis of the adenovirus E1B-55K-anchored proteome reveals its link to ubiquitination machinery.J Virol. 2002 Sep;76(18):9194-206. doi: 10.1128/jvi.76.18.9194-9206.2002. J Virol. 2002. PMID: 12186903 Free PMC article.
-
The role of NK cells in oncolytic viral therapy: a focus on hepatocellular carcinoma.J Transl Genet Genom. 2021;5:304-322. doi: 10.20517/jtgg.2021.27. Epub 2021 Aug 4. J Transl Genet Genom. 2021. PMID: 34888493 Free PMC article.
References
-
- Arrigo S J, Chen I S Y. Rev is necessary for translation but not cytoplasmic accumulation of HIV-1 vif, vpr, and env/vpu 2 RNAs. Genes Dev. 1991;5:808–819. - PubMed
-
- Baker S J, Markowitz S, Fearon E R, Willson J K, Vogelstein B. Suppression of human colorectal carcinoma cell growth by wild-type p53. Science. 1990;249:912–915. - PubMed
-
- Barker D D, Berk A J. Adenovirus proteins from both E1B reading frames are required for transformation of rodent cells by viral infection and DNA transfection. Virology. 1988;56:107–121. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

