Role of phosphorylation of Alzheimer's amyloid precursor protein during neuronal differentiation
- PMID: 10341243
- PMCID: PMC6782598
- DOI: 10.1523/JNEUROSCI.19-11-04421.1999
Role of phosphorylation of Alzheimer's amyloid precursor protein during neuronal differentiation
Abstract
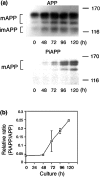
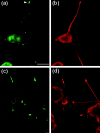
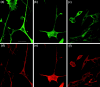
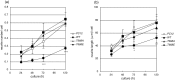
Alzheimer's amyloid precursor protein (APP), the precursor of beta-amyloid (Abeta), is an integral membrane protein with a receptor-like structure. We recently demonstrated that the mature APP (mAPP; N- and O-glycosylated form) is phosphorylated at Thr668 (numbering for APP695 isoform), specifically in neurons. Phosphorylation of mAPP appears to occur during, and after, neuronal differentiation. Here we report that the phosphorylation of mAPP begins 48-72 hr after treatment of PC12 cells with NGF and that this correlates with the timing of neurite outgrowth. The phosphorylated form of APP is distributed in neurites and mostly in the growth cones of differentiating PC12 cells. PC12 cells stably expressing APP with Thr668Glu substitution showed remarkably reduced neurite extension after treatment with NGF. These observations suggest that the phosphorylated form of APP may play an important role in neurite outgrowth of differentiating neurons.
Figures

Similar articles
-
The cytoplasmic domain of Alzheimer's amyloid precursor protein is phosphorylated at Thr654, Ser655, and Thr668 in adult rat brain and cultured cells.Mol Med. 1997 Feb;3(2):111-23. Mol Med. 1997. PMID: 9085254 Free PMC article.
-
NGF controls APP cleavage by downregulating APP phosphorylation at Thr668: relevance for Alzheimer's disease.Aging Cell. 2016 Aug;15(4):661-72. doi: 10.1111/acel.12473. Epub 2016 Apr 13. Aging Cell. 2016. PMID: 27076121 Free PMC article.
-
Phosphorylation of amyloid precursor protein (APP) at Thr668 regulates the nuclear translocation of the APP intracellular domain and induces neurodegeneration.Mol Cell Biol. 2006 Jun;26(11):4327-38. doi: 10.1128/MCB.02393-05. Mol Cell Biol. 2006. PMID: 16705182 Free PMC article.
-
Insights into the physiological function of the β-amyloid precursor protein: beyond Alzheimer's disease.J Neurochem. 2014 Jun;129(5):756-69. doi: 10.1111/jnc.12675. Epub 2014 Mar 7. J Neurochem. 2014. PMID: 24517464 Free PMC article. Review.
-
Mechanism of glial differentiation of neural progenitor cells by amyloid precursor protein.Neurodegener Dis. 2008;5(3-4):170-2. doi: 10.1159/000113693. Epub 2008 Mar 6. Neurodegener Dis. 2008. PMID: 18322381 Review.
Cited by
-
The amyloid-beta precursor protein is phosphorylated via distinct pathways during differentiation, mitosis, stress, and degeneration.Mol Biol Cell. 2007 Oct;18(10):3835-44. doi: 10.1091/mbc.e06-07-0625. Epub 2007 Jul 18. Mol Biol Cell. 2007. PMID: 17634293 Free PMC article.
-
The novel protein MANI modulates neurogenesis and neurite-cone growth.J Cell Mol Med. 2011 Aug;15(8):1713-25. doi: 10.1111/j.1582-4934.2010.01134.x. J Cell Mol Med. 2011. PMID: 20716133 Free PMC article.
-
P60TRP interferes with the GPCR/secretase pathway to mediate neuronal survival and synaptogenesis.J Cell Mol Med. 2011 Nov;15(11):2462-77. doi: 10.1111/j.1582-4934.2010.01248.x. J Cell Mol Med. 2011. PMID: 21199326 Free PMC article.
-
Elucidation of O-glycosylation structures of the beta-amyloid precursor protein by liquid chromatography-mass spectrometry using electron transfer dissociation and collision induced dissociation.J Proteome Res. 2009 Feb;8(2):631-42. doi: 10.1021/pr800758g. J Proteome Res. 2009. PMID: 19093876 Free PMC article.
-
Pro-inflammatory interleukin-18 increases Alzheimer's disease-associated amyloid-β production in human neuron-like cells.J Neuroinflammation. 2012 Aug 16;9:199. doi: 10.1186/1742-2094-9-199. J Neuroinflammation. 2012. PMID: 22898493 Free PMC article.
References
-
- Beher D, Hesse L, Masters CL, Multhaup G. Regulation of amyloid protein precursor (APP) binding to collagen and mapping of the binding sites on APP and collagen type 1. J Biol Chem. 1996;271:1613–1620. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
