Atrial natriuretic peptide-stimulated Ca2+ reabsorption in rabbit kidney requires membrane-targeted, cGMP-dependent protein kinase type II
- PMID: 10339545
- PMCID: PMC26839
- DOI: 10.1073/pnas.96.11.6084
Atrial natriuretic peptide-stimulated Ca2+ reabsorption in rabbit kidney requires membrane-targeted, cGMP-dependent protein kinase type II
Abstract
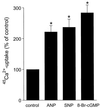
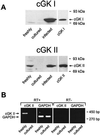
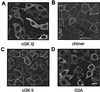
Atrial natriuretic peptide (ANP) and nitric oxide (NO) are key regulators of ion and water transport in the kidney. Here, we report that these cGMP-elevating hormones stimulate Ca2+ reabsorption via a novel mechanism specifically involving type II cGMP-dependent protein kinase (cGK II). ANP and the NO donor, sodium nitroprusside (SNP), markedly increased Ca2+ uptake in freshly immunodissected rabbit connecting tubules (CNT) and cortical collecting ducts (CCD). Although readily increasing cGMP, ANP and SNP did not affect Ca2+ and Na+ reabsorption in primary cultures of these segments. Immunoblot analysis demonstrated that cGK II, and not cGK I, was present in freshly isolated CNT and CCD but underwent a complete down-regulation during the primary cell culture. However, upon adenoviral reexpression of cGK II in primary cultures, ANP, SNP, and 8-Br-cGMP readily increased Ca2+ reabsorption. In contrast, no cGMP-dependent effect on electrogenic Na+ transport was observed. The membrane localization of cGK II proved to be crucial for its action, because a nonmyristoylated cGK II mutant that was shown to be localized in the cytosol failed to mediate ANP-stimulated Ca2+ transport. The Ca2+-regulatory function of cGK II appeared isotype-specific because no cGMP-mediated increase in Ca2+ transport was observed after expression of the cytosolic cGK Ibeta or a membrane-bound cGK II/Ibeta chimer. These results demonstrate that ANP- and NO-stimulated Ca2+ reabsorption requires membrane-targeted cGK II.
Figures
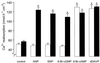

Similar articles
-
Heterogeneous nuclear ribonucleoprotein A1 is a novel cellular target of atrial natriuretic peptide signaling in renal epithelial cells.Cell Signal. 2012 May;24(5):1100-8. doi: 10.1016/j.cellsig.2012.01.006. Epub 2012 Jan 17. Cell Signal. 2012. PMID: 22285803 Free PMC article.
-
Hormone-stimulated Ca2+ reabsorption in rabbit kidney cortical collecting system is cAMP-independent and involves a phorbol ester-insensitive PKC isotype.Kidney Int. 1999 Jan;55(1):225-33. doi: 10.1046/j.1523-1755.1999.00228.x. Kidney Int. 1999. PMID: 9893131
-
Expression of type II cGMP-dependent protein kinase in rat kidney is regulated by dehydration and correlated with renin gene expression.J Clin Invest. 1996 Aug 1;98(3):662-70. doi: 10.1172/JCI118837. J Clin Invest. 1996. PMID: 8698857 Free PMC article.
-
Renal actions of atrial natriuretic peptide: regulation of collecting duct sodium and water transport.Annu Rev Physiol. 1990;52:747-59. doi: 10.1146/annurev.ph.52.030190.003531. Annu Rev Physiol. 1990. PMID: 2139559 Review.
-
Regulation of collecting duct Na+ reabsorption by ANP 31-67.Clin Exp Pharmacol Physiol. 1995 Feb;22(2):121-4. doi: 10.1111/j.1440-1681.1995.tb01967.x. Clin Exp Pharmacol Physiol. 1995. PMID: 7621604 Review.
Cited by
-
Calbindin-D28K dynamically controls TRPV5-mediated Ca2+ transport.EMBO J. 2006 Jul 12;25(13):2978-88. doi: 10.1038/sj.emboj.7601186. Epub 2006 Jun 8. EMBO J. 2006. PMID: 16763551 Free PMC article.
-
B-type natriuretic peptide overexpression ameliorates hepatorenal fibrocystic disease in a rat model of polycystic kidney disease.Kidney Int. 2017 Sep;92(3):657-668. doi: 10.1016/j.kint.2017.02.017. Epub 2017 Apr 14. Kidney Int. 2017. PMID: 28416225 Free PMC article.
-
Direct interaction with Rab11a targets the epithelial Ca2+ channels TRPV5 and TRPV6 to the plasma membrane.Mol Cell Biol. 2006 Jan;26(1):303-12. doi: 10.1128/MCB.26.1.303-312.2006. Mol Cell Biol. 2006. PMID: 16354700 Free PMC article.
-
Cyclic gmp is a measure of physiologic stress.J Natl Med Assoc. 2001 Jul-Aug;93(7-8):256-62. J Natl Med Assoc. 2001. PMID: 11491275 Free PMC article.
-
ENaC is regulated by natriuretic peptide receptor-dependent cGMP signaling.Am J Physiol Renal Physiol. 2013 Apr 1;304(7):F930-7. doi: 10.1152/ajprenal.00638.2012. Epub 2013 Jan 16. Am J Physiol Renal Physiol. 2013. PMID: 23324181 Free PMC article.
References
-
- Bindels R J M, Hartog A, Timmermans J A H, Van Os C H. Am J Physiol. 1991;261:F799–F807. - PubMed
-
- Friedman P A, Gesek F A. Physiol Rev. 1995;75:429–471. - PubMed
-
- Van Baal J, Yu A, Hartog A, Willems P H G M, Lytton J, Bindels R J M. Am J Physiol. 1996;271:F985–F993. - PubMed
-
- Lau K, Bourdeau J E. J Biol Chem. 1989;264:4028–4032. - PubMed
-
- Hoenderop J G J, Hartog A, Willems P H G M, Bindels R J M. Am J Physiol. 1998;43:F736–F743. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

