Sulfotransferases of two specificities function in the reconstitution of high endothelial cell ligands for L-selectin
- PMID: 10330415
- PMCID: PMC2133194
- DOI: 10.1083/jcb.145.4.899
Sulfotransferases of two specificities function in the reconstitution of high endothelial cell ligands for L-selectin
Abstract
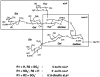
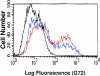
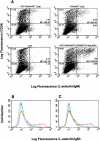
L-selectin, a lectin-like receptor, mediates rolling of lymphocytes on high endothelial venules (HEVs) in secondary lymphoid organs by interacting with HEV ligands. These ligands consist of a complex of sialomucins, candidates for which are glycosylation- dependent cell adhesion molecule 1 (GlyCAM-1), CD34, and podocalyxin. The ligands must be sialylated, fucosylated, and sulfated for optimal recognition by L-selectin. Our previous structural characterization of GlyCAM-1 has demonstrated two sulfation modifications, Gal-6-sulfate and GlcNAc-6-sulfate in the context of sialyl Lewis x. We now report the cloning of a Gal-6-sulfotransferase and a GlcNAc-6-sulfotransferase, which can modify GlyCAM-1 and CD34. The Gal-6-sulfotransferase shows a wide tissue distribution. In contrast, the GlcNAc-6-sulfotransferase is highly restricted to HEVs, as revealed by Northern analysis and in situ hybridization. Expression of either enzyme in Chinese hamster ovary cells, along with CD34 and fucosyltransferase VII, results in ligand activity, as detected by binding of an L-selectin/IgM chimera. When coexpressed, the two sulfotransferases synergize to produce strongly enhanced chimera binding.
Figures





Similar articles
-
A novel, high endothelial venule-specific sulfotransferase expresses 6-sulfo sialyl Lewis(x), an L-selectin ligand displayed by CD34.Immunity. 1999 Jul;11(1):79-89. doi: 10.1016/s1074-7613(00)80083-7. Immunity. 1999. PMID: 10435581
-
Sulfation of a high endothelial venule-expressed ligand for L-selectin. Effects on tethering and rolling of lymphocytes.J Exp Med. 1999 Oct 4;190(7):935-42. doi: 10.1084/jem.190.7.935. J Exp Med. 1999. PMID: 10510083 Free PMC article.
-
Significant decrease in alpha1,3-linked fucose in association with increase in 6-sulfated N-acetylglucosamine in peripheral lymph node addressin of FucT-VII-deficient mice exhibiting diminished lymphocyte homing.Glycobiology. 2007 Mar;17(3):277-93. doi: 10.1093/glycob/cwl077. Epub 2006 Dec 15. Glycobiology. 2007. PMID: 17172261
-
Biosynthesis of sulfated L-selectin ligands in human high endothelial venules (HEV).Adv Exp Med Biol. 1998;435:55-62. doi: 10.1007/978-1-4615-5383-0_6. Adv Exp Med Biol. 1998. PMID: 9498065 Review.
-
Carbohydrate sulfotransferases in lymphocyte homing.Glycobiology. 2000 Sep;10(9):849-56. doi: 10.1093/glycob/10.9.849. Glycobiology. 2000. PMID: 10988246 Review.
Cited by
-
Two distinct lymphocyte homing systems involved in the pathogenesis of chronic inflammatory gastrointestinal diseases.Semin Immunopathol. 2012 May;34(3):401-13. doi: 10.1007/s00281-012-0302-3. Epub 2012 May 10. Semin Immunopathol. 2012. PMID: 22572886 Review.
-
Apical membrane expression of distinct sulfated glycans represents a novel marker of cholangiolocellular carcinoma.Lab Invest. 2016 Dec;96(12):1246-1255. doi: 10.1038/labinvest.2016.104. Epub 2016 Oct 17. Lab Invest. 2016. PMID: 27748735
-
Metabolic glycoengineering: sialic acid and beyond.Glycobiology. 2009 Dec;19(12):1382-401. doi: 10.1093/glycob/cwp115. Epub 2009 Aug 12. Glycobiology. 2009. PMID: 19675091 Free PMC article. Review.
-
Lymphocyte-HEV interactions in lymph nodes of a sulfotransferase-deficient mouse.J Exp Med. 2003 Nov 3;198(9):1289-300. doi: 10.1084/jem.20030057. J Exp Med. 2003. PMID: 14597732 Free PMC article.
-
Lymphotoxin plays a crucial role in the development and function of nasal-associated lymphoid tissue through regulation of chemokines and peripheral node addressin.Am J Pathol. 2005 Jan;166(1):135-46. doi: 10.1016/S0002-9440(10)62239-0. Am J Pathol. 2005. PMID: 15632007 Free PMC article.
References
-
- Andrews P, Milsom D, Ford W. Migration of lymphocytes across specialized vascular endothelium. V. Production of a sulphated macromolecule by high endothelial cells in lymph nodes. J Cell Sci. 1982;57:277–292. - PubMed
-
- Baumhueter S, Dybdal N, Kyle C, Lasky LA. Global vascular expression of murine CD34, a sialomucin-like endothelial ligand for L-selectin. Blood. 1994;84:2554–2565. - PubMed
-
- Baumhueter S, Singer MS, Henzel W, Hemmerich S, Renz M, Rosen SD, Lasky LA. Binding of L-selectin to the vascular sialomucin, CD34. Science. 1993;262:436–438. - PubMed
-
- Berg EL, McEvoy LM, Berlin C, Bargatze RF, Butcher EC. L-selectin-mediated lymphocyte rolling on MAdCAM-1. Nature. 1993;366:695–698. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

