Sequences between the enhancer and promoter in the long terminal repeat affect murine leukemia virus pathogenicity and replication in the thymus
- PMID: 10233950
- PMCID: PMC112532
- DOI: 10.1128/JVI.73.6.4890-4898.1999
Sequences between the enhancer and promoter in the long terminal repeat affect murine leukemia virus pathogenicity and replication in the thymus
Abstract
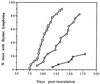
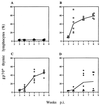
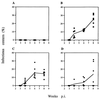
We previously showed that the 93-bp region between the enhancer and promoter (named DEN for downstream of enhancer) of the long terminal repeat (LTR) of the MCF13 murine leukemia virus is an important determinant of the ability of this virus to induce thymic lymphoma. In this study we observed that DEN plays a role in the regulation of virus replication in the thymus during the preleukemic period. A NF-kappaB site in the DEN region partially contributes to the effect of DEN on both lymphomagenicity and virus replication. To further study the effects of DEN and the NF-kappaB site on viral pathogenicity during the preleukemic period, we examined replication of wild-type and mutant viruses with a deletion of the NF-kappaB site or the entire DEN region in the thymus. Thymic lymphocytes which were infected with wild-type and mutant viruses were predominantly the CD3(-) CD4(+) CD8(+) and CD3(+) CD4(+) CD8(+) cells. The increase in infection by wild-type virus and both mutant viruses of these two subpopulations during the preleukemic period ranged from 9- to 84-fold, depending upon the time point and virus. The major difference between the wild-type and both mutant viruses was the lower rate and lower level of mutant virus replication in these thymic subpopulations. Significant differences in replication between wild-type and both mutant viruses were seen in the CD3(-) CD4(+) CD8(+) and CD3(-) CD4(-) CD8(-) subpopulations, suggesting that these thymic cell types are important targets for viral transformation.
Figures
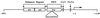
Similar articles
-
Role of the LTR region between the enhancer and promoter in mink cell focus-forming murine leukemia virus pathogenesis.Virology. 2001 Apr 25;283(1):121-31. doi: 10.1006/viro.2001.0879. Virology. 2001. PMID: 11312668
-
Contributions to transcriptional activity and to viral leukemogenicity made by sequences within and downstream of the MCF13 murine leukemia virus enhancer.J Virol. 1992 Dec;66(12):7080-8. doi: 10.1128/JVI.66.12.7080-7088.1992. J Virol. 1992. PMID: 1331510 Free PMC article.
-
Identification of a region of a murine leukemia virus long terminal repeat with novel transcriptional regulatory activities.J Virol. 1994 May;68(5):3308-16. doi: 10.1128/JVI.68.5.3308-3316.1994. J Virol. 1994. PMID: 8151791 Free PMC article.
-
Viral pathogenesis and immunity within the thymus.Immunol Res. 1998;17(1-2):75-82. doi: 10.1007/BF02786432. Immunol Res. 1998. PMID: 9479569 Review.
-
Murine leukemia viruses with recombinant env genes: a discussion of their role in leukemogenesis.Curr Top Microbiol Immunol. 1983;103:75-108. doi: 10.1007/978-3-642-68943-7_4. Curr Top Microbiol Immunol. 1983. PMID: 6303710 Review. No abstract available.
Cited by
-
Tolerance has its limits: how the thymus copes with infection.Trends Immunol. 2013 Oct;34(10):502-10. doi: 10.1016/j.it.2013.06.004. Epub 2013 Jul 16. Trends Immunol. 2013. PMID: 23871487 Free PMC article. Review.
-
Differential cell killing by lymphomagenic murine leukemia viruses occurs independently of p53 activation and mitochondrial damage.J Virol. 2004 May;78(10):5088-96. doi: 10.1128/jvi.78.10.5088-5096.2004. J Virol. 2004. PMID: 15113890 Free PMC article.
-
NF-kappaB activation stimulates transcription and replication of retrovirus XMRV in human B-lineage and prostate carcinoma cells.J Virol. 2011 Apr;85(7):3179-86. doi: 10.1128/JVI.02333-10. Epub 2011 Jan 26. J Virol. 2011. PMID: 21270144 Free PMC article.
-
Long terminal repeat regions from exogenous but not endogenous feline leukemia viruses transactivate cellular gene expression.J Virol. 2000 Oct;74(20):9742-8. doi: 10.1128/jvi.74.20.9742-9748.2000. J Virol. 2000. PMID: 11000248 Free PMC article.
-
Sequence analysis of porcine endogenous retrovirus long terminal repeats and identification of transcriptional regulatory regions.J Virol. 2003 Jan;77(1):142-9. doi: 10.1128/jvi.77.1.142-149.2003. J Virol. 2003. PMID: 12477819 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

