Proline residues in human immunodeficiency virus type 1 p6(Gag) exert a cell type-dependent effect on viral replication and virion incorporation of Pol proteins
- PMID: 10233929
- PMCID: PMC112511
- DOI: 10.1128/JVI.73.6.4696-4704.1999
Proline residues in human immunodeficiency virus type 1 p6(Gag) exert a cell type-dependent effect on viral replication and virion incorporation of Pol proteins
Abstract
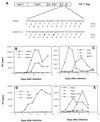
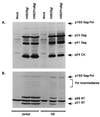
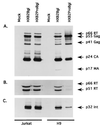
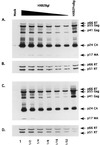
The C terminus of the HIV-1 Gag protein contains a proline-rich domain termed p6(Gag). This domain has been shown to play a role in efficient virus release and incorporation of Vpr into virions. In a previous study (X. F. Yu, L. Dawson, C. J. Tian, C. Flexner, and M. Dettenhofer, J. Virol. 72:3412-3417, 1998), we observed that the removal of the p6 domain of Gag as well as drastic mutations in the PTAP motif resulted in reduced virion-associated Pol proteins from transfected COS cells. In the present study, amino acid substitutions at residues 5 and 7 of p6(Gag) resulted in a cell type-dependent replication of the mutant virus in CD4(+) T cells; the virus was replication competent in Jurkat cells but restricted in H9 cells and primary blood-derived monocytes. Established Jurkat and H9 cell lines expressing p6(Gag) mutant and parental virus were used to further understand this defect. Mutant virions produced from H9 cells, which displayed no defect in extracellular virion production, showed an approximately 16-fold reduction in Pol protein levels, whereas the levels of Pol proteins were only marginally reduced in mutant virions produced from Jurkat cells. The reduction in the virion-associated Pol proteins could not be accounted for by differences in the levels of intracellular p160(Gag-Pol) or in the interaction between p55(Gag) and p160(Gag-Pol) precursors. Electron microscopic analysis of the p6(Gag) mutant virions showed a predominately immature morphology in the absence of significant defects in Gag proteolytic cleavage. Taken together, these data suggest that the proline-rich motif of p6(Gag) is involved in the late stages of virus maturation, which include the packaging of cleaved Pol proteins in viral particles, a process which may involve cell-type-specific factors.
Figures


Similar articles
-
Mutations of the human immunodeficiency virus type 1 p6Gag domain result in reduced retention of Pol proteins during virus assembly.J Virol. 1998 Apr;72(4):3412-7. doi: 10.1128/JVI.72.4.3412-3417.1998. J Virol. 1998. PMID: 9525672 Free PMC article.
-
Role of the C terminus Gag protein in human immunodeficiency virus type 1 virion assembly and maturation.J Gen Virol. 1995 Dec;76 ( Pt 12):3171-9. doi: 10.1099/0022-1317-76-12-3171. J Gen Virol. 1995. PMID: 8847526
-
Incorporation of functional human immunodeficiency virus type 1 integrase into virions independent of the Gag-Pol precursor protein.J Virol. 1997 Oct;71(10):7704-10. doi: 10.1128/JVI.71.10.7704-7710.1997. J Virol. 1997. PMID: 9311854 Free PMC article.
-
Exploring HIV-1 Maturation: A New Frontier in Antiviral Development.Viruses. 2024 Sep 6;16(9):1423. doi: 10.3390/v16091423. Viruses. 2024. PMID: 39339899 Free PMC article. Review.
-
The choreography of HIV-1 proteolytic processing and virion assembly.J Biol Chem. 2012 Nov 30;287(49):40867-74. doi: 10.1074/jbc.R112.399444. Epub 2012 Oct 5. J Biol Chem. 2012. PMID: 23043111 Free PMC article. Review.
Cited by
-
Viral late domains.J Virol. 2002 May;76(10):4679-87. doi: 10.1128/jvi.76.10.4679-4687.2002. J Virol. 2002. PMID: 11967285 Free PMC article. Review. No abstract available.
-
Targeting human immunodeficiency virus type 1 assembly, maturation and budding.Drug Target Insights. 2007;2:159-82. Epub 2007 Jul 20. Drug Target Insights. 2007. PMID: 21901072 Free PMC article.
-
Partial restoration of replication of simian immunodeficiency virus by point mutations in either the dimerization initiation site (DIS) or Gag region after deletion mutagenesis within the DIS.J Virol. 2001 Dec;75(23):11920-3. doi: 10.1128/JVI.75.23.11920-11923.2001. J Virol. 2001. PMID: 11689677 Free PMC article.
-
Budding of a Retrovirus: Some Assemblies Required.Viruses. 2020 Oct 20;12(10):1188. doi: 10.3390/v12101188. Viruses. 2020. PMID: 33092109 Free PMC article. Review.
-
The late-domain-containing protein p6 is the predominant phosphoprotein of human immunodeficiency virus type 1 particles.J Virol. 2002 Feb;76(3):1015-24. doi: 10.1128/jvi.76.3.1015-1024.2002. J Virol. 2002. PMID: 11773377 Free PMC article.
References
-
- Ansari-Lari M A, Donehower L A, Gibbs R A. Analysis of human immunodeficiency virus type 1 integrase mutants. Virology. 1995;213:680. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

