The role of human T-lymphotropic virus type 1 (HTLV-1)-infected dendritic cells in the development of HTLV-1-associated myelopathy/tropical spastic paraparesis
- PMID: 10233916
- PMCID: PMC112498
- DOI: 10.1128/JVI.73.6.4575-4581.1999
The role of human T-lymphotropic virus type 1 (HTLV-1)-infected dendritic cells in the development of HTLV-1-associated myelopathy/tropical spastic paraparesis
Abstract
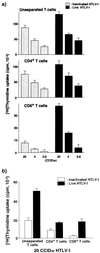
The development of human T-lymphotropic virus type 1 (HTLV-1)-associated myelopathy/tropical spastic paraparesis (HAM/TSP) is closely associated with the activation of T cells which are HTLV-1 specific but may cross-react with neural antigens (Ags). Immature dendritic cells (DCs), differentiated from normal donor monocytes by using recombinant granulocyte-macrophage colony-stimulating factor and recombinant interleukin-4, were pulsed with HTLV-1 in vitro. The pulsed DCs contained HTLV-1 proviral DNA and expressed HTLV-1 Gag Ag on their surface 6 days after infection. The DCs matured by lipopolysaccharides stimulated autologous CD4(+) T cells and CD8(+) T cells in a viral dose-dependent manner. However, the proliferation level of CD4(+) T cells was five- to sixfold higher than that of CD8(+) T cells. In contrast to virus-infected DCs, DCs pulsed with heat-inactivated virions activated only CD4(+) T cells. To clarify the role of DCs in HAM/TSP development, monocytes from patients were cultured for 4 days in the presence of the cytokines. The expression of CD86 Ag on DCs was higher and that of CD1a Ag was more down-regulated than in DCs generated from normal monocytes. DCs from two of five patients expressed HTLV-1 Gag Ag. Furthermore, both CD4(+) and CD8(+) T cells from the patients were greatly stimulated by contact with autologous DCs pulsed with inactivated viral Ag as well as HTLV-1-infected DCs. These results suggest that DCs are susceptible to HTLV-1 infection and that their cognate interaction with T cells may contribute to the development of HAM/TSP.
Figures


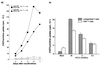


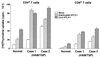
Similar articles
-
Marked suppression of T cells by a benzothiophene derivative in patients with human T-lymphotropic virus type I-associated myelopathy/tropical spastic paraparesis.Clin Diagn Lab Immunol. 1999 May;6(3):316-22. doi: 10.1128/CDLI.6.3.316-322.1999. Clin Diagn Lab Immunol. 1999. PMID: 10225829 Free PMC article.
-
Demonstration of human T lymphotropic virus type I (HTLV-I) tax-specific CD8+ lymphocytes directly in peripheral blood of HTLV-I-associated myelopathy/tropical spastic paraparesis patients by intracellular cytokine detection.J Immunol. 1998 Jul 1;161(1):482-8. J Immunol. 1998. PMID: 9647259
-
Dendritic Cells Pulsed with HAM/TSP Exosomes Sensitize CD4 T Cells to Enhance HTLV-1 Infection, Induce Helper T-Cell Polarization, and Decrease Cytotoxic T-Cell Response.Viruses. 2024 Sep 10;16(9):1443. doi: 10.3390/v16091443. Viruses. 2024. PMID: 39339919 Free PMC article.
-
[HTLV-I--associated myelopathy/tropical spastic paraparesis (HAM/TSP)].Ryoikibetsu Shokogun Shirizu. 2000;(31):9-12. Ryoikibetsu Shokogun Shirizu. 2000. PMID: 11269197 Review. Japanese. No abstract available.
-
HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) inflammatory network.Inflamm Allergy Drug Targets. 2008 Jun;7(2):98-107. doi: 10.2174/187152808785107642. Inflamm Allergy Drug Targets. 2008. PMID: 18691139 Review.
Cited by
-
Peripheral blood mononuclear cells from individuals infected with human T-cell lymphotropic virus type 1 have a reduced capacity to respond to recall antigens.Clin Vaccine Immunol. 2006 May;13(5):547-52. doi: 10.1128/CVI.13.5.547-552.2006. Clin Vaccine Immunol. 2006. PMID: 16682474 Free PMC article. Clinical Trial.
-
Molecular Mechanisms of Neurodegenerative Diseases Induced by Human Retroviruses: A Review.Am J Infect Dis. 2009 Jul 1;5(3):231-258. doi: 10.3844/ajidsp.2009.231.258. Am J Infect Dis. 2009. PMID: 20352020 Free PMC article.
-
The tug-of-war between dendritic cells and human chronic viruses.Int Rev Immunol. 2011 Oct-Dec;30(5-6):341-65. doi: 10.3109/08830185.2011.561506. Int Rev Immunol. 2011. PMID: 22053973 Free PMC article. Review.
-
The combination of IκB kinase β inhibitor and everolimus modulates expression of interleukin-10 in human T-cell lymphotropic virus type-1-infected T cells.Immunology. 2013 Mar;138(3):216-27. doi: 10.1111/imm.12035. Immunology. 2013. PMID: 23278479 Free PMC article.
-
Mycobacterium leprae infection in monocyte-derived dendritic cells and its influence on antigen-presenting function.Infect Immun. 2002 Sep;70(9):5167-76. doi: 10.1128/IAI.70.9.5167-5176.2002. Infect Immun. 2002. PMID: 12183567 Free PMC article.
References
-
- Cameron P U, Fredenthal P S, Barker J M, Gezelter S, Inaba K, Steinman R M. Dendritic cells exposed to human immunodeficiency virus type-1 transmit a vigorous cytopathic infection to CD4+ T cells. Science (Washington, DC) 1992;257:383–387. - PubMed
-
- Cameron P U, Pope M, Gezelter S, Steinman R M. Infection and apoptotic cell death of CD4+ T cells during an immune response to HIV-1 pulsed dedritic cells. AIDS Res Hum Retroviruses. 1994;10:61–71. - PubMed
-
- Dhib-Jalbut S, Hoffman P M, Yamabe T, Sun D, Xia J, Eisenberg H, Bergey G, Ruscetti F W. Extracellular human T-cell lymphotropic virus type I Tax protein induces cytokine production in adult human microglial cells. Ann Neurol. 1994;36:787–790. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

