Phosphorylation of adducin by Rho-kinase plays a crucial role in cell motility
- PMID: 10209029
- PMCID: PMC2133101
- DOI: 10.1083/jcb.145.2.347
Phosphorylation of adducin by Rho-kinase plays a crucial role in cell motility
Abstract
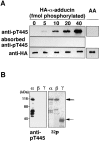
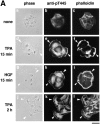
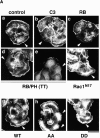
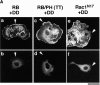
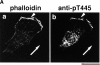
Adducin is a membrane skeletal protein that binds to actin filaments (F-actin) and thereby promotes the association of spectrin with F-actin to form a spectrin-actin meshwork beneath plasma membranes such as ruffling membranes. Rho-associated kinase (Rho- kinase), which is activated by the small guanosine triphosphatase Rho, phosphorylates alpha-adducin and thereby enhances the F-actin-binding activity of alpha-adducin in vitro. Here we identified the sites of phosphorylation of alpha-adducin by Rho-kinase as Thr445 and Thr480. We prepared antibody that specifically recognized alpha-adducin phosphorylated at Thr445, and found by use of this antibody that Rho-kinase phosphorylated alpha-adducin at Thr445 in COS7 cells in a Rho-dependent manner. Phosphorylated alpha-adducin accumulated in the membrane ruffling area of Madin-Darby canine kidney (MDCK) epithelial cells and the leading edge of scattering cells during the action of tetradecanoylphorbol-13-acetate (TPA) or hepatocyte growth factor (HGF). The microinjection of Botulinum C3 ADP-ribosyl-transferase, dominant negative Rho-kinase, or alpha-adducinT445A,T480A (substitution of Thr445 and Thr480 by Ala) inhibited the TPA-induced membrane ruffling in MDCK cells and wound-induced migration in NRK49F cells. alpha-AdducinT445D,T480D (substitution of Thr445 and Thr480 by Asp), but not alpha-adducinT445A,T480A, counteracted the inhibitory effect of the dominant negative Rho-kinase on the TPA-induced membrane ruffling in MDCK cells. Taken together, these results indicate that Rho-kinase phosphorylates alpha-adducin downstream of Rho in vivo, and that the phosphorylation of adducin by Rho-kinase plays a crucial role in the regulation of membrane ruffling and cell motility.
Figures









Similar articles
-
Phosphorylation of myosin-binding subunit (MBS) of myosin phosphatase by Rho-kinase in vivo.J Cell Biol. 1999 Nov 29;147(5):1023-38. doi: 10.1083/jcb.147.5.1023. J Cell Biol. 1999. PMID: 10579722 Free PMC article.
-
Adducin is an in vivo substrate for protein kinase C: phosphorylation in the MARCKS-related domain inhibits activity in promoting spectrin-actin complexes and occurs in many cells, including dendritic spines of neurons.J Cell Biol. 1998 Jul 27;142(2):485-97. doi: 10.1083/jcb.142.2.485. J Cell Biol. 1998. PMID: 9679146 Free PMC article.
-
Regulation of the association of adducin with actin filaments by Rho-associated kinase (Rho-kinase) and myosin phosphatase.J Biol Chem. 1998 Mar 6;273(10):5542-8. doi: 10.1074/jbc.273.10.5542. J Biol Chem. 1998. PMID: 9488679
-
Activation of moesin and adducin by Rho-kinase downstream of Rho.Biophys Chem. 1999 Dec 13;82(2-3):139-47. doi: 10.1016/s0301-4622(99)00113-1. Biophys Chem. 1999. PMID: 10631797 Review.
-
Adducin: structure, function and regulation.Cell Mol Life Sci. 2000 Jun;57(6):884-95. doi: 10.1007/PL00000731. Cell Mol Life Sci. 2000. PMID: 10950304 Free PMC article. Review.
Cited by
-
Physiological role of ROCKs in the cardiovascular system.Am J Physiol Cell Physiol. 2006 Mar;290(3):C661-8. doi: 10.1152/ajpcell.00459.2005. Am J Physiol Cell Physiol. 2006. PMID: 16469861 Free PMC article. Review.
-
Identification and characterization of Aplysia adducin, an Aplysia cytoskeletal protein homologous to mammalian adducins: increased phosphorylation at a protein kinase C consensus site during long-term synaptic facilitation.J Neurosci. 2003 Apr 1;23(7):2675-85. doi: 10.1523/JNEUROSCI.23-07-02675.2003. J Neurosci. 2003. PMID: 12684453 Free PMC article.
-
Alpha-adducin dissociates from F-actin and spectrin during platelet activation.J Cell Biol. 2003 May 12;161(3):557-70. doi: 10.1083/jcb.200211122. J Cell Biol. 2003. PMID: 12743105 Free PMC article.
-
Adducins inhibit lung cancer cell migration through mechanisms involving regulation of cell-matrix adhesion and cadherin-11 expression.Biochim Biophys Acta Mol Cell Res. 2019 Mar;1866(3):395-408. doi: 10.1016/j.bbamcr.2018.10.001. Epub 2018 Oct 2. Biochim Biophys Acta Mol Cell Res. 2019. PMID: 30290240 Free PMC article.
-
Molecular parameters of head and neck cancer metastasis.Crit Rev Eukaryot Gene Expr. 2011;21(2):143-53. doi: 10.1615/critreveukargeneexpr.v21.i2.40. Crit Rev Eukaryot Gene Expr. 2011. PMID: 22077153 Free PMC article. Review.
References
-
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J Biol Chem. 1996a;271:20246–20249. - PubMed
-
- Amano M, Mukai H, Ono Y, Chihara K, Matsui T, Hamajima Y, Okawa K, Iwamatsu A, Kaibuchi K. Identification of a putative target for Rho as a serine-threonine kinase, PKN. Science. 1996b;271:648–650. - PubMed
-
- Amano M, Chihara K, Kimura K, Fukata Y, Nakamura N, Matsuura Y, Kaibuchi K. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science. 1997;275:1308–1311. - PubMed
-
- Amano M, Chihara K, Nakamura N, Fukata Y, Yano T, Shibata M, Ikebe M, Kaibuchi K. Myosin II activation promotes neurite retraction during the action of Rho and Rho-kinase. Genes Cells. 1998;3:177–188. - PubMed
-
- Bennett V, Gardner K, Steiner JP. Brain adducin: a protein kinase C substrate that may mediate site-directed assembly at the spectrin-actin junction. J Biol Chem. 1988;263:5860–5869. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

