A novel mechanism of ion homeostasis and salt tolerance in yeast: the Hal4 and Hal5 protein kinases modulate the Trk1-Trk2 potassium transporter
- PMID: 10207057
- PMCID: PMC84126
- DOI: 10.1128/MCB.19.5.3328
A novel mechanism of ion homeostasis and salt tolerance in yeast: the Hal4 and Hal5 protein kinases modulate the Trk1-Trk2 potassium transporter
Abstract
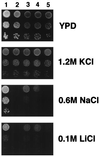
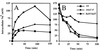
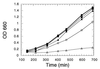
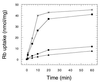
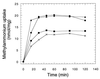
The regulation of intracellular ion concentrations is a fundamental property of living cells. Although many ion transporters have been identified, the systems that modulate their activity remain largely unknown. We have characterized two partially redundant genes from Saccharomyces cerevisiae, HAL4/SAT4 and HAL5, that encode homologous protein kinases implicated in the regulation of cation uptake. Overexpression of these genes increases the tolerance of yeast cells to sodium and lithium, whereas gene disruptions result in greater cation sensitivity. These phenotypic effects of the mutations correlate with changes in cation uptake and are dependent on a functional Trk1-Trk2 potassium transport system. In addition, hal4 hal5 and trk1 trk2 mutants exhibit similar phenotypes: (i) they are deficient in potassium uptake; (ii) their growth is sensitive to a variety of toxic cations, including lithium, sodium, calcium, tetramethylammonium, hygromycin B, and low pH; and (iii) they exhibit increased uptake of methylammonium, an indicator of membrane potential. These results suggest that the Hal4 and Hal5 protein kinases activate the Trk1-Trk2 potassium transporter, increasing the influx of potassium and decreasing the membrane potential. The resulting loss in electrical driving force reduces the uptake of toxic cations and improves salt tolerance. Our data support a role for regulation of membrane potential in adaptation to salt stress that is mediated by the Hal4 and Hal5 kinases.
Figures

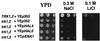

Similar articles
-
Regulation of yeast H(+)-ATPase by protein kinases belonging to a family dedicated to activation of plasma membrane transporters.Mol Cell Biol. 2000 Oct;20(20):7654-61. doi: 10.1128/MCB.20.20.7654-7661.2000. Mol Cell Biol. 2000. PMID: 11003661 Free PMC article.
-
Key role for intracellular K+ and protein kinases Sat4/Hal4 and Hal5 in the plasma membrane stabilization of yeast nutrient transporters.Mol Cell Biol. 2007 Aug;27(16):5725-36. doi: 10.1128/MCB.01375-06. Epub 2007 Jun 4. Mol Cell Biol. 2007. PMID: 17548466 Free PMC article.
-
TRK2 is not a low-affinity potassium transporter in Saccharomyces cerevisiae.J Bacteriol. 1994 Jan;176(1):249-52. doi: 10.1128/jb.176.1.249-252.1994. J Bacteriol. 1994. PMID: 8282703 Free PMC article.
-
Monovalent cation transporters at the plasma membrane in yeasts.Yeast. 2019 Apr;36(4):177-193. doi: 10.1002/yea.3355. Epub 2018 Oct 3. Yeast. 2019. PMID: 30193006 Review.
-
[Potassium transport in yeast].Rev Latinoam Microbiol. 1999 Apr-Jun;41(2):91-103. Rev Latinoam Microbiol. 1999. PMID: 10970213 Review. Spanish.
Cited by
-
Coordination of K+ transporters in neurospora: TRK1 is scarce and constitutive, while HAK1 is abundant and highly regulated.Eukaryot Cell. 2013 May;12(5):684-96. doi: 10.1128/EC.00017-13. Epub 2013 Mar 8. Eukaryot Cell. 2013. PMID: 23475706 Free PMC article.
-
Two cation transporters Ena1 and Nha1 cooperatively modulate ion homeostasis, antifungal drug resistance, and virulence of Cryptococcus neoformans via the HOG pathway.Fungal Genet Biol. 2012 Apr;49(4):332-45. doi: 10.1016/j.fgb.2012.02.001. Epub 2012 Feb 11. Fungal Genet Biol. 2012. PMID: 22343280 Free PMC article.
-
Response of fission yeast to toxic cations involves cooperative action of the stress-activated protein kinase Spc1/Sty1 and the Hal4 protein kinase.Mol Cell Biol. 2005 May;25(10):3945-55. doi: 10.1128/MCB.25.10.3945-3955.2005. Mol Cell Biol. 2005. PMID: 15870269 Free PMC article.
-
A genomewide screen for tolerance to cationic drugs reveals genes important for potassium homeostasis in Saccharomyces cerevisiae.Eukaryot Cell. 2011 Sep;10(9):1241-50. doi: 10.1128/EC.05029-11. Epub 2011 Jul 1. Eukaryot Cell. 2011. PMID: 21724935 Free PMC article.
-
Widespread mRNA association with cytoskeletal motor proteins and identification and dynamics of myosin-associated mRNAs in S. cerevisiae.PLoS One. 2012;7(2):e31912. doi: 10.1371/journal.pone.0031912. Epub 2012 Feb 16. PLoS One. 2012. PMID: 22359641 Free PMC article.
References
-
- Alepuz P M, Cunningham K W, Estruch F. Glucose repression affects ion homeostasis in yeast through the regulation of the stress-activated ENA1 gene. Mol Microbiol. 1997;26:91–98. - PubMed
-
- André B. An overview of membrane transport proteins in Saccharomyces cerevisiae. Yeast. 1995;11:1575–1611. - PubMed
-
- Benito B, Portillo F, Lagunas R. In vivo activation of the yeast plasma membrane ATPase during nitrogen starvation. Identification of the regulatory domain that controls activation. FEBS Lett. 1992;300:271–274. - PubMed
-
- Broach J R, Strathern J N, Hicks J B. Transformation in yeast: development of a hybrid cloning vector and isolation of the CAN1 gene. Gene. 1979;8:121–133. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
