A mutation in the heterotrimeric stimulatory guanine nucleotide binding protein alpha-subunit with impaired receptor-mediated activation because of elevated GTPase activity
- PMID: 10200251
- PMCID: PMC16321
- DOI: 10.1073/pnas.96.8.4268
A mutation in the heterotrimeric stimulatory guanine nucleotide binding protein alpha-subunit with impaired receptor-mediated activation because of elevated GTPase activity
Abstract
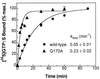
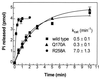
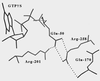
It has been reported that substitution of Arg258, a residue within the GTPase domain of the heterotrimeric guanine nucleotide binding protein (G protein) alpha-subunit (alphas), to alanine (alphas-R258A) results in decreased activation by receptor or aluminum fluoride (AlF4-) and increased basal GDP release. Arg258 interacts with Gln170 in the helical domain, and, presumably, loss of this interaction between the GTPase and helical domain leads to more rapid GDP release, resulting in decreased activation by AlF4- and increased thermolability. In this study, we mutate Gln170 to alanine (alphas-Q170A) and demonstrate that this mutant, like alphas-R258A, has decreased activation by AlF4-, increased thermolability (both reversed in the presence of excess guanine nucleotide), and an increased rate of GDP release. However, unlike alphas-R258A, alphas-Q170A does not have impaired receptor-mediated activation. Therefore, this interdomain interaction is critical to maintain normal guanine nucleotide binding (and hence normal activation by AlF4-) but is not important for receptor-mediated activation. In single turnover GTPase assays, the catalytic rate for GTP hydrolysis of alphas-R258A was 14-fold higher than normal whereas that of alphas-Q170A was unaffected. Examination of the alphas crystal structure suggests that Arg258, through interactions with Glu50, might constrain the position of Arg201, a residue critical for catalyzing the GTPase reaction. This is an example of a mutation in a heterotrimeric G protein that results in an increased intrinsic GTPase activity and provides another mechanism by which G protein mutations can impair signal transduction.
Figures

Similar articles
-
A novel mutation in the switch 3 region of Gsalpha in a patient with Albright hereditary osteodystrophy impairs GDP binding and receptor activation.J Biol Chem. 1998 Sep 11;273(37):23976-83. doi: 10.1074/jbc.273.37.23976. J Biol Chem. 1998. PMID: 9727013
-
Mutagenesis of the conserved residue Glu259 of Gsalpha demonstrates the importance of interactions between switches 2 and 3 for activation.J Biol Chem. 1999 Feb 19;274(8):4977-84. doi: 10.1074/jbc.274.8.4977. J Biol Chem. 1999. PMID: 9988742
-
Conditional activation defect of a human Gsalpha mutant.Proc Natl Acad Sci U S A. 1997 May 27;94(11):5656-61. doi: 10.1073/pnas.94.11.5656. Proc Natl Acad Sci U S A. 1997. PMID: 9159128 Free PMC article.
-
S111N mutation in the helical domain of human Gs(alpha) reduces its GDP/GTP exchange rate.J Cell Biochem. 2002;85(3):615-20. doi: 10.1002/jcb.10128. J Cell Biochem. 2002. PMID: 11968001
-
Mutations at the domain interface of GSalpha impair receptor-mediated activation by altering receptor and guanine nucleotide binding.J Biol Chem. 1998 Jun 12;273(24):15053-60. doi: 10.1074/jbc.273.24.15053. J Biol Chem. 1998. PMID: 9614114
Cited by
-
Biarsenical ligands bind to endogenous G-protein α-subunits and enable allosteric sensing of nucleotide binding.BMC Biochem. 2013 Dec 17;14:37. doi: 10.1186/1471-2091-14-37. BMC Biochem. 2013. PMID: 24344803 Free PMC article.
-
Influence of sequential guanidinium methylation on the energetics of the guanidinium...guanine dimer and guanidinium...guanine...cytosine trimer: implications for the control of protein...DNA interactions by arginine methyltransferases.J Phys Chem B. 2008 Dec 25;112(51):16995-7002. doi: 10.1021/jp808288p. J Phys Chem B. 2008. PMID: 19368013 Free PMC article.
-
A structural determinant that renders G alpha(i) sensitive to activation by GIV/girdin is required to promote cell migration.J Biol Chem. 2010 Apr 23;285(17):12765-77. doi: 10.1074/jbc.M109.045161. Epub 2010 Feb 15. J Biol Chem. 2010. PMID: 20157114 Free PMC article.
-
Fibrous Dysplasia of Temporal Bone.Indian J Otolaryngol Head Neck Surg. 2022 Dec;74(Suppl 3):4350-4355. doi: 10.1007/s12070-021-03009-6. Epub 2022 Jan 10. Indian J Otolaryngol Head Neck Surg. 2022. PMID: 36742801 Free PMC article.
-
Structure and dynamics of GPCR signaling complexes.Nat Struct Mol Biol. 2018 Jan;25(1):4-12. doi: 10.1038/s41594-017-0011-7. Epub 2018 Jan 8. Nat Struct Mol Biol. 2018. PMID: 29323277 Free PMC article. Review.
References
-
- Spiegel A M, Shenker A, Weinstein L S. Endocr Rev. 1992;13:536–565. - PubMed
-
- Neer E J. Cell. 1995;80:249–257. - PubMed
-
- Birnbaumer L, Abramowitz J, Brown A M. Biochim Biophys Acta. 1990;1031:163–224. - PubMed
-
- Yatani A, Codina J, Imoto Y, Reeves J P, Birnbaumer L, Brown A M. Science. 1987;238:1288–1292. - PubMed
-
- Schreibmayer W, Dessauer W, Vorobiev D, Gilman A G, Lester H A, Davidson N, Dascal N. Nature (London) 1996;380:624–627. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

