Activation of promoter P4 of the autonomous parvovirus minute virus of mice at early S phase is required for productive infection
- PMID: 10196282
- PMCID: PMC104165
- DOI: 10.1128/JVI.73.5.3877-3885.1999
Activation of promoter P4 of the autonomous parvovirus minute virus of mice at early S phase is required for productive infection
Abstract
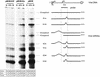
Autonomous parvoviruses are tightly dependent on host cell factors for various steps of their life cycle. In particular, DNA replication and gene expression of the prototype strain of the minute virus of mice (MVMp) are closely linked to the onset of host cell DNA replication, pointing to the involvement of an S-phase-specific cellular factor(s) in parvovirus multiplication. The viral nonstructural protein NS-1 is absolutely required for parvovirus DNA replication and is able to transcriptionally regulate parvoviral and heterologous promoters. We previously showed that the promoter P4, which directs the transcription unit encoding the NS proteins, is activated at the onset of S phase. This activation is dependent on an E2F motif in the proximal region of promoter P4. An infectious MVM DNA clone was mutated in the E2F motif of P4. The wild type and the E2F mutant derivative were tested for their ability to produce progeny viruses after transfection of permissive cells. In the context of the whole MVMp genome, the E2F mutation abolished P4 induction in S phase and inactivated the infectious molecular clone, which failed to become amplified and generate progeny particles. The virus could be rescued when NS proteins were supplied in trans, showing that P4 hyperactivity in S is needed to reach a level of NS-1 expression that is sufficient to drive the viral replication cycle. These data show that E2F-mediated P4 activation at the early S phase is a limiting factor for parvovirus production. The primary barrier to parvovirus gene expression in G1 is thought to be promoter formation rather than activation, due to the poor conversion of the parental single-strand genome to a duplex form. The S dependence of P4 activation may therefore be a sign of the virus adaptation to life in the S-phase host cell. If the conversion block in G1 were to be leaky, the S induction of promoter P4 could be envisioned as a safeguard against the production of toxic NS proteins until cells reach the S phase and provide the full machinery for parvovirus replication.
Figures



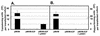
Similar articles
-
Genome replication and postencapsidation functions mapping to the nonstructural gene restrict the host range of a murine parvovirus in human cells.J Virol. 2001 Dec;75(23):11573-82. doi: 10.1128/JVI.75.23.11573-11582.2001. J Virol. 2001. PMID: 11689639 Free PMC article.
-
The MVMp P4 promoter is a host cell-type range determinant in vivo.Virology. 2017 Jun;506:141-151. doi: 10.1016/j.virol.2017.03.012. Epub 2017 Apr 6. Virology. 2017. PMID: 28391161
-
Comparison of promoter activity in Aleutian mink disease parvovirus, minute virus of mice, and canine parvovirus: possible role of weak promoters in the pathogenesis of Aleutian mink disease parvovirus infection.J Virol. 1993 Apr;67(4):1877-86. doi: 10.1128/JVI.67.4.1877-1886.1993. J Virol. 1993. PMID: 8383215 Free PMC article.
-
Structure and function of the parvoviral NS1 protein: a review.Virus Genes. 2023 Apr;59(2):195-203. doi: 10.1007/s11262-022-01944-2. Epub 2022 Oct 17. Virus Genes. 2023. PMID: 36253516 Review.
-
Parvovirus replication.Microbiol Rev. 1990 Sep;54(3):316-29. doi: 10.1128/mr.54.3.316-329.1990. Microbiol Rev. 1990. PMID: 2215424 Free PMC article. Review.
Cited by
-
Activation of a helper and not regulatory human CD4+ T cell response by oncolytic H-1 parvovirus.PLoS One. 2012;7(2):e32197. doi: 10.1371/journal.pone.0032197. Epub 2012 Feb 16. PLoS One. 2012. PMID: 22359669 Free PMC article.
-
Replicating parvoviruses that target colon cancer cells.J Virol. 2003 Jun;77(12):6683-91. doi: 10.1128/jvi.77.12.6683-6691.2003. J Virol. 2003. PMID: 12767988 Free PMC article.
-
Human parvovirus B19 infection causes cell cycle arrest of human erythroid progenitors at late S phase that favors viral DNA replication.J Virol. 2013 Dec;87(23):12766-75. doi: 10.1128/JVI.02333-13. Epub 2013 Sep 18. J Virol. 2013. PMID: 24049177 Free PMC article.
-
Structure of the NS1 protein N-terminal origin recognition/nickase domain from the emerging human bocavirus.J Virol. 2013 Nov;87(21):11487-93. doi: 10.1128/JVI.01770-13. Epub 2013 Aug 21. J Virol. 2013. PMID: 23966383 Free PMC article.
-
Tumor Selectivity of Oncolytic Parvoviruses: From in vitro and Animal Models to Cancer Patients.Front Bioeng Biotechnol. 2015 Apr 22;3:55. doi: 10.3389/fbioe.2015.00055. eCollection 2015. Front Bioeng Biotechnol. 2015. PMID: 25954743 Free PMC article. Review.
References
-
- Beijersbergen R L, Bernards R. Cell cycle regulation by the retinoblastoma family of growth inhibitory proteins. Biochim Biophys Acta. 1996;1287:103–120. - PubMed
-
- Chaney W G, Howard D R, Pollard J W, Sallustio S, Stanley P. High-frequency transfection of CHO cells using polybrene. Somat Cell Mol Genet. 1986;12:237–244. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

