Scar, a WASp-related protein, activates nucleation of actin filaments by the Arp2/3 complex
- PMID: 10097107
- PMCID: PMC22364
- DOI: 10.1073/pnas.96.7.3739
Scar, a WASp-related protein, activates nucleation of actin filaments by the Arp2/3 complex
Abstract
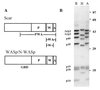
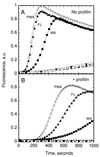
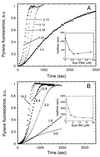
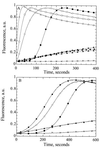
The Arp2/3 complex, a stable assembly of two actin-related proteins (Arp2 and Arp3) with five other subunits, caps the pointed end of actin filaments and nucleates actin polymerization with low efficiency. WASp and Scar are two similar proteins that bind the p21 subunit of the Arp2/3 complex, but their effect on the nucleation activity of the complex was not known. We report that full-length, recombinant human Scar protein, as well as N-terminally truncated Scar proteins, enhance nucleation by the Arp2/3 complex. By themselves, these proteins either have no effect or inhibit actin polymerization. The actin monomer-binding W domain and the p21-binding A domain from the C terminus of Scar are both required to activate Arp2/3 complex. A proline-rich domain in the middle of Scar enhances the activity of the W and A domains. Preincubating Scar and Arp2/3 complex with actin filaments overcomes the initial lag in polymerization, suggesting that efficient nucleation by the Arp2/3 complex requires assembly on the side of a preexisting filament-a dendritic nucleation mechanism. The Arp2/3 complex with full-length Scar, Scar containing P, W, and A domains, or Scar containing W and A domains overcomes inhibition of nucleation by the actin monomer-binding protein profilin, giving active nucleation over a low background of spontaneous nucleation. These results show that Scar and, likely, related proteins, such as the Cdc42 targets WASp and N-WASp, are endogenous activators of actin polymerization by the Arp2/3 complex.
Figures

Similar articles
-
Direct observation of dendritic actin filament networks nucleated by Arp2/3 complex and WASP/Scar proteins.Nature. 2000 Apr 27;404(6781):1007-11. doi: 10.1038/35010008. Nature. 2000. PMID: 10801131
-
Different WASP family proteins stimulate different Arp2/3 complex-dependent actin-nucleating activities.Curr Biol. 2001 Dec 11;11(24):1903-13. doi: 10.1016/s0960-9822(01)00603-0. Curr Biol. 2001. PMID: 11747816
-
Influence of the C terminus of Wiskott-Aldrich syndrome protein (WASp) and the Arp2/3 complex on actin polymerization.Biochemistry. 1999 Nov 16;38(46):15212-22. doi: 10.1021/bi991843+. Biochemistry. 1999. PMID: 10563804
-
Signalling to actin assembly via the WASP (Wiskott-Aldrich syndrome protein)-family proteins and the Arp2/3 complex.Biochem J. 2004 May 15;380(Pt 1):1-17. doi: 10.1042/BJ20040176. Biochem J. 2004. PMID: 15040784 Free PMC article. Review.
-
Regulation of actin filament network formation through ARP2/3 complex: activation by a diverse array of proteins.Annu Rev Biochem. 2001;70:649-76. doi: 10.1146/annurev.biochem.70.1.649. Annu Rev Biochem. 2001. PMID: 11395419 Review.
Cited by
-
Profilin regulates F-actin network homeostasis by favoring formin over Arp2/3 complex.Dev Cell. 2015 Jan 12;32(1):43-53. doi: 10.1016/j.devcel.2014.10.027. Epub 2014 Dec 24. Dev Cell. 2015. PMID: 25543282 Free PMC article.
-
Polarized branched Actin modulates cortical mechanics to produce unequal-size daughters during asymmetric division.Nat Cell Biol. 2023 Feb;25(2):235-245. doi: 10.1038/s41556-022-01058-9. Epub 2023 Feb 6. Nat Cell Biol. 2023. PMID: 36747081 Free PMC article.
-
Molecular basis of the intracellular spreading of Shigella.Infect Immun. 2001 Oct;69(10):5959-66. doi: 10.1128/IAI.69.10.5959-5966.2001. Infect Immun. 2001. PMID: 11553531 Free PMC article. Review. No abstract available.
-
CYRI/ Fam49 Proteins Represent a New Class of Rac1 Interactors.Commun Integr Biol. 2019 Jul 23;12(1):112-118. doi: 10.1080/19420889.2019.1643665. eCollection 2019. Commun Integr Biol. 2019. PMID: 31413787 Free PMC article.
-
Two tandem verprolin homology domains are necessary for a strong activation of Arp2/3 complex-induced actin polymerization and induction of microspike formation by N-WASP.Proc Natl Acad Sci U S A. 2000 Nov 7;97(23):12631-6. doi: 10.1073/pnas.190351397. Proc Natl Acad Sci U S A. 2000. PMID: 11058146 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

