Targeted disruption of fibronectin-integrin interactions in human gingival fibroblasts by the RI protease of Porphyromonas gingivalis W50
- PMID: 10085025
- PMCID: PMC96535
- DOI: 10.1128/IAI.67.4.1837-1843.1999
Targeted disruption of fibronectin-integrin interactions in human gingival fibroblasts by the RI protease of Porphyromonas gingivalis W50
Abstract
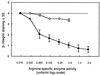
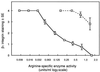
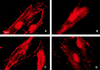
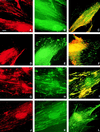
Cell surface integrins mediate interactions between cells and their extracellular matrix and are frequently exploited by a range of bacterial pathogens to facilitate adherence and/or invasion. In this study we examined the effects of Porphyromonas gingivalis proteases on human gingival fibroblast (HGF) integrins and their fibronectin matrix. Culture supernatant from the virulent strain W50 caused considerably greater loss of the beta1 integrin subunit from HGF in vitro than did that of the beige-pigmented strain W50/BE1. Prior treatment of the W50 culture supernatant with the protease inhibitor Nalpha-p-tosyl-L-lysine chloromethyl ketone (TLCK) blocked its effects on cultured cells, indicating that this process is proteolytically mediated. Purified arginine-specific proteases from P. gingivalis W50 were able to mimic the effects of the whole-culture supernatant on loss of beta1 integrin expression. However purified RI, an alpha/beta heterodimer in which the catalytic chain is associated with an adhesin chain, was 12 times more active than RIA, the catalytic monomer, in causing loss of the alpha5beta1 integrin (fibronectin receptor) from HGF. No effect was observed on the alphaVbeta3 integrin (vitronectin receptor). The sites of action of RI and RIA were investigated in cells exposed to proteases pretreated with TLCK to inactivate the catalytic component. Use of both monoclonal antibody 1A1, which recognizes only the adhesin chain of RI, and a rabbit antibody against P. gingivalis whole cells indicated localization of RI on the fibroblasts in a clear, linear pattern typical of that seen with fibronectin and alpha5beta1 integrin. Exact colocalization of RI with fibronectin and its alpha5beta1 receptor was confirmed by double labeling and multiple-exposure photomicroscopy. In contrast, RIA bound to fibroblasts in a weak, patchy manner, showing only fine linear or granular staining. It is concluded that the adhesin component of RI targets the P. gingivalis arginine-protease to sites of fibronectin deposition on HGF, contributing to the rapid loss of both fibronectin and its main alpha5beta1 integrin receptor. Given the importance of integrin-ligand interactions in fibroblast function, their targeted disruption by RI may represent a novel mechanism of damage in periodontal disease.
Figures

Similar articles
-
Nuclear targeting of Porphyromonas gingivalis W50 protease in epithelial cells.Infect Immun. 2002 Oct;70(10):5740-50. doi: 10.1128/IAI.70.10.5740-5750.2002. Infect Immun. 2002. PMID: 12228304 Free PMC article.
-
Altered expression and modification of proteases from an avirulent mutant of Porphyromonas gingivalis W50 (W50/BE1).Microbiology (Reading). 1998 Sep;144 ( Pt 9):2487-2496. doi: 10.1099/00221287-144-9-2487. Microbiology (Reading). 1998. PMID: 9782496
-
The prpR1 and prR2 arginine-specific protease genes of Porphyromonas gingivalis W50 produce five biochemically distinct enzymes.Mol Microbiol. 1997 Mar;23(5):955-65. doi: 10.1046/j.1365-2958.1997.2831647.x. Mol Microbiol. 1997. PMID: 9076732
-
Molecular interaction of Porphyromonas gingivalis with host cells: implication for the microbial pathogenesis of periodontal disease.J Periodontol. 2003 Jan;74(1):90-6. doi: 10.1902/jop.2003.74.1.90. J Periodontol. 2003. PMID: 12593602 Review.
-
Porphyromonas gingivalis gingipains: the molecular teeth of a microbial vampire.Curr Protein Pept Sci. 2003 Dec;4(6):409-26. doi: 10.2174/1389203033487009. Curr Protein Pept Sci. 2003. PMID: 14683427 Review.
Cited by
-
Gingipains from Porphyromonas gingivalis W83 induce cell adhesion molecule cleavage and apoptosis in endothelial cells.Infect Immun. 2005 Mar;73(3):1543-52. doi: 10.1128/IAI.73.3.1543-1552.2005. Infect Immun. 2005. PMID: 15731052 Free PMC article.
-
Translocation of Porphyromonas gingivalis gingipain adhesin peptide A44 to host mitochondria prevents apoptosis.Infect Immun. 2010 Aug;78(8):3616-24. doi: 10.1128/IAI.00187-10. Epub 2010 Jun 14. Infect Immun. 2010. PMID: 20547744 Free PMC article.
-
Nuclear targeting of Porphyromonas gingivalis W50 protease in epithelial cells.Infect Immun. 2002 Oct;70(10):5740-50. doi: 10.1128/IAI.70.10.5740-5750.2002. Infect Immun. 2002. PMID: 12228304 Free PMC article.
-
Activation of blood coagulation factor IX by gingipains R, arginine-specific cysteine proteinases from Porphyromonas gingivalis.Biochem J. 2001 Jan 15;353(Pt 2):325-31. doi: 10.1042/0264-6021:3530325. Biochem J. 2001. PMID: 11139397 Free PMC article.
-
Porphyromonas gingivalis gingipains and adhesion to epithelial cells.Infect Immun. 2001 May;69(5):3048-56. doi: 10.1128/IAI.69.5.3048-3056.2001. Infect Immun. 2001. PMID: 11292723 Free PMC article.
References
-
- Birkedal-Hansen H, Taylor R E, Zambon J J, Barwa P K, Neiders M E. Characterization of collagenolytic activity from strains of Bacteroides gingivalis. J Periodontal Res. 1988;23:258–264. - PubMed
-
- Birkedal-Hansen H, Wells B R, Lin H Y, Caulfield P W, Taylor R E. Activation of keratinocyte-mediated collagen (type I) breakdown by suspected human periodontopathogen. Evidence of a novel mechanism of connective tissue breakdown. J Periodontal Res. 1984;19:645–650. - PubMed
-
- Collinson L M, Rangarajan M, Curtis M A. Altered expression and modification of proteases from an avirulent mutant of Porphyromonas gingivalis W50 (W50/BE1) Microbiology. 1998;144:2487–2496. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

