The J domain of papovaviral large tumor antigen is required for synergistic interaction with the POU-domain protein Tst-1/Oct6/SCIP
- PMID: 10082511
- PMCID: PMC84038
- DOI: 10.1128/MCB.19.4.2455
The J domain of papovaviral large tumor antigen is required for synergistic interaction with the POU-domain protein Tst-1/Oct6/SCIP
Abstract
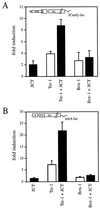
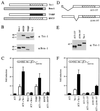
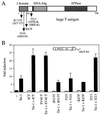
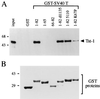
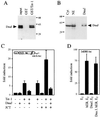
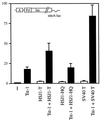
Large T antigens from polyomaviruses are multifunctional proteins with roles in transcriptional regulation, viral DNA replication, and cellular transformation. They have been shown to enhance the activity of various cellular transcription factors. In the case of the POU protein Tst-1/Oct6/SCIP, this enhancement involves a direct physical interaction between the POU domain of the transcription factor and the amino-terminal region of large T antigen. Here we have analyzed the structural requirements for synergistic interaction between the two proteins in greater detail. Tst-1/Oct6/SCIP and the related POU protein Brn-1 were both capable of direct physical interaction with large T antigen. Nevertheless, only Tst-1/Oct6/SCIP functioned synergistically with large T antigen. This differential behavior was due to differences in the amino-terminal regions of the proteins, as evident from chimeras between Tst-1/Oct6/SCIP and Brn-1. Synergy was specifically observed for constructs containing the amino-terminal region of Tst-1/Oct6/SCIP. Large T antigen, on the other hand, functioned synergistically with Tst-1/Oct6/SCIP only when the integrity of its J-domain-containing amino terminus was maintained. Mutations that disrupted the J domain concomitantly abolished the ability to enhance the function of Tst-1/Oct6/SCIP. The J domain of T antigen was also responsible for the physical interaction with Tst-1/Oct6/SCIP and could be replaced in this property by other J domains. Intriguingly, a heterologous J domain from a human DnaJ protein partially substituted for the amino terminus of T antigen even with regard to the synergistic enhancement of Tst-1/Oct6/SCIP function. Given the general role of J domains, we propose chaperone activity as the underlying mechanism for synergy between Tst-1/Oct6/SCIP and large T antigens.
Figures

Similar articles
-
Redundancy of class III POU proteins in the oligodendrocyte lineage.J Biol Chem. 1997 Dec 19;272(51):32286-93. doi: 10.1074/jbc.272.51.32286. J Biol Chem. 1997. PMID: 9405434
-
T antigen of human papovavirus JC stimulates transcription of the POU domain factor Tst-1/Oct6/SCIP.DNA Cell Biol. 1996 Dec;15(12):1057-62. doi: 10.1089/dna.1996.15.1057. DNA Cell Biol. 1996. PMID: 8985119
-
The POU domain protein Tst-1 and papovaviral large tumor antigen function synergistically to stimulate glia-specific gene expression of JC virus.Proc Natl Acad Sci U S A. 1994 Jul 5;91(14):6433-7. doi: 10.1073/pnas.91.14.6433. Proc Natl Acad Sci U S A. 1994. PMID: 8022800 Free PMC article.
-
Regulation of DNA virus transcription by cellular POU family transcription factors.Rev Med Virol. 1999 Jan-Mar;9(1):31-8. doi: 10.1002/(sici)1099-1654(199901/03)9:1<31::aid-rmv231>3.0.co;2-2. Rev Med Virol. 1999. PMID: 10371670 Review.
-
POU domain factors in neural development.Adv Exp Med Biol. 1998;449:39-53. doi: 10.1007/978-1-4615-4871-3_4. Adv Exp Med Biol. 1998. PMID: 10026784 Review.
Cited by
-
The DnaJ proteins DJA6 and DJA5 are essential for chloroplast iron-sulfur cluster biogenesis.EMBO J. 2021 Jul 1;40(13):e106742. doi: 10.15252/embj.2020106742. Epub 2021 Apr 15. EMBO J. 2021. PMID: 33855718 Free PMC article.
-
Inhibition of Simian Virus 40 replication by targeting the molecular chaperone function and ATPase activity of T antigen.Virus Res. 2009 Apr;141(1):71-80. doi: 10.1016/j.virusres.2008.12.018. Epub 2009 Feb 4. Virus Res. 2009. PMID: 19200446 Free PMC article.
-
New insights on human polyomavirus JC and pathogenesis of progressive multifocal leukoencephalopathy.Clin Dev Immunol. 2013;2013:839719. doi: 10.1155/2013/839719. Epub 2013 Apr 17. Clin Dev Immunol. 2013. PMID: 23690827 Free PMC article. Review.
-
JC virus T' proteins encoded by alternatively spliced early mRNAs enhance T antigen-mediated viral DNA replication in human cells.J Neurovirol. 2001 Jun;7(3):250-64. doi: 10.1080/13550280152403290. J Neurovirol. 2001. PMID: 11517399
-
Simian virus 40 T antigens and J domains: analysis of Hsp40 cochaperone functions in Escherichia coli.J Virol. 2003 Oct;77(19):10706-13. doi: 10.1128/jvi.77.19.10706-10713.2003. J Virol. 2003. PMID: 12970459 Free PMC article.
References
-
- Alvarez B G, Rosenfeld M G, Swanson L W. Model of forebrain regionalization based on spatiotemporal patterns of POU-III homeobox gene expression, birthdates, and morphological features. J Comp Neurol. 1995;355:237–295. - PubMed
-
- Berger L, Smith D B, Davidson I, Hwang J-J, Fanning E, Wildeman A G. Interaction between T antigen and TEA domain of the factor TEF-1 derepresses simian virus 40 late promoter in vitro: identification of T-antigen domains important for transcriptional control. J Virol. 1996;70:1203–1212. - PMC - PubMed
-
- Bermingham J R, Scherer S S, O’Connell S, Arroyo E, Kalla K A, Powell F L, Rosenfeld M G. Tst-1/Oct6/SCIP regulates a unique step in peripheral myelination and is required for normal respiration. Genes Dev. 1996;10:1751–1762. - PubMed
-
- Campbell K S, Mullane K P, Aksoy I A, Stubdal H, Zalvide J, Pipas J M, Silver P A, Roberts T M, Schaffhausen B S, DeCaprio J A. DnaJ/hsp40 chaperone domain of SV40 large T antigen promotes efficient DNA replication. Genes Dev. 1997;11:1098–1110. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
