Human antibody responses to mature and immature forms of viral envelope in respiratory syncytial virus infection: significance for subunit vaccines
- PMID: 10074145
- PMCID: PMC104055
- DOI: 10.1128/JVI.73.4.2956-2962.1999
Human antibody responses to mature and immature forms of viral envelope in respiratory syncytial virus infection: significance for subunit vaccines
Abstract
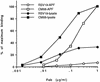
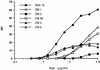
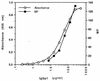
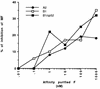
A number of antibodies generated during human respiratory syncytial virus (RSV) infection have been cloned by the phage library approach. Antibodies reactive with an immunodominant epitope on the F glycoprotein of this virus have a high affinity for affinity-purified F antigen. These antibodies, however, have a much lower affinity for mature F glycoprotein on the surface of infected cells and are nonneutralizing. In contrast, a potent neutralizing antibody has a high affinity for mature F protein but a much lower affinity for purified F protein or F protein in viral lysates. The data indicate that at least two F protein immunogens are produced during natural RSV infection: immature F, found in viral lysates, and mature F, found on infected cells or virions. Binding studies with polyclonal human immunoglobulin G suggest that the antibody responses to the two immunogens are of similar magnitudes. Competitive binding studies suggest that overlap between the responses is relatively limited. A mature envelope with an antigenic configuration different from that of the immature envelope has an evolutionary advantage in that the infecting virus is less subject to neutralization by the humoral response to the immature envelope that inevitably arises following lysis of infected cells. Subunit vaccines may be at a disadvantage because they most often resemble immature envelope molecules and ignore this aspect of viral evasion.
Figures
Similar articles
-
Effect of Previous Respiratory Syncytial Virus Infection on Murine Immune Responses to F and G Protein-Containing Virus-Like Particles.J Virol. 2019 Apr 17;93(9):e00087-19. doi: 10.1128/JVI.00087-19. Print 2019 May 1. J Virol. 2019. PMID: 30760576 Free PMC article.
-
Antibody responses against epitopes on the F protein of bovine respiratory syncytial virus differ in infected or vaccinated cattle.Arch Virol. 1997;142(11):2195-210. doi: 10.1007/s007050050235. Arch Virol. 1997. PMID: 9672586
-
Antigenic Fingerprinting of Respiratory Syncytial Virus (RSV)-A-Infected Hematopoietic Cell Transplant Recipients Reveals Importance of Mucosal Anti-RSV G Antibodies in Control of RSV Infection in Humans.J Infect Dis. 2020 Feb 3;221(4):636-646. doi: 10.1093/infdis/jiz608. J Infect Dis. 2020. PMID: 31745552 Free PMC article.
-
Human respiratory syncytial virus: pathogenesis, immune responses, and current vaccine approaches.Eur J Clin Microbiol Infect Dis. 2018 Oct;37(10):1817-1827. doi: 10.1007/s10096-018-3289-4. Epub 2018 Jun 6. Eur J Clin Microbiol Infect Dis. 2018. PMID: 29876771 Review.
-
Antigenic diversity of respiratory syncytial viruses and its implication for immunoprophylaxis in ruminants.Vet Microbiol. 1993 Nov;37(3-4):319-41. doi: 10.1016/0378-1135(93)90032-3. Vet Microbiol. 1993. PMID: 8116189 Review.
Cited by
-
Enhanced Neutralizing Antibody Response Induced by Respiratory Syncytial Virus Prefusion F Protein Expressed by a Vaccine Candidate.J Virol. 2015 Sep;89(18):9499-510. doi: 10.1128/JVI.01373-15. Epub 2015 Jul 8. J Virol. 2015. PMID: 26157122 Free PMC article.
-
Human metapneumovirus fusion protein vaccines that are immunogenic and protective in cotton rats.J Virol. 2007 Jan;81(2):698-707. doi: 10.1128/JVI.00844-06. Epub 2006 Oct 18. J Virol. 2007. PMID: 17050599 Free PMC article.
-
Sensitive and Specific Detection of Low-Level Antibody Responses in Mild Middle East Respiratory Syndrome Coronavirus Infections.Emerg Infect Dis. 2019 Oct;25(10):1868-1877. doi: 10.3201/eid2510.190051. Epub 2019 Oct 17. Emerg Infect Dis. 2019. PMID: 31423970 Free PMC article.
-
Genetic and structural determinants of virus neutralizing antibodies.Immunol Res. 2001;23(2-3):135-45. doi: 10.1385/IR:23:2-3:135. Immunol Res. 2001. PMID: 11444379 Review.
-
Antibodies in human infectious disease.Immunol Res. 2000;21(2-3):265-78. doi: 10.1385/IR:21:2-3:265. Immunol Res. 2000. PMID: 10852127 Review.
References
-
- Barbas C F, Collet T A, Amberg W, Roben P, Binley J M, Hoekstra D, Cababa D, Jones T M, Williamson R A, Pilkington G R, Haigwood N L, Cabezas E, Satterthwait A C, Sanz I, Burton D R. Molecular profile of an antibody response to HIV-1 as probed by combinatorial libraries. J Mol Biol. 1993;230:812–823. - PubMed
-
- Barbas C F, III, Crowe J E, Jr, Cababa D, Jones T M, Zebedee S L, Murphy B R, Chanock R M, Burton D R. Human monoclonal Fab fragments derived from a combinatorial library bind to respiratory syncytial virus F glycoprotein and neutralize infectivity. Proc Natl Acad Sci USA. 1992;89:10164–10168. - PMC - PubMed
-
- Berman P W, Gray A M, Wrin T, Vennari J C, Eastman D J, Nakamura G R, Francis D P, Gorse G, Schwartz D H. Genetic and immunologic characterization of viruses infecting MN-rgp120-vaccinated volunteers. J Infect Dis. 1997;176:384–397. - PubMed
-
- Bourgeois C, Corvaisier C, Bour J B, Kohli E, Pothier P. Use of synthetic peptides to locate neutralizing antigenic domains on the fusion protein of respiratory syncytial virus. J Gen Virol. 1991;72:1051–1058. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

