Insulin-like growth factor-1 induces Mdm2 and down-regulates p53, attenuating the myocyte renin-angiotensin system and stretch-mediated apoptosis
- PMID: 10027414
- PMCID: PMC1850006
- DOI: 10.1016/S0002-9440(10)65302-3
Insulin-like growth factor-1 induces Mdm2 and down-regulates p53, attenuating the myocyte renin-angiotensin system and stretch-mediated apoptosis
Abstract
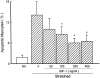
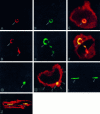
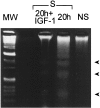
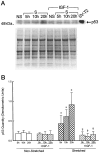
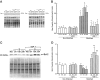
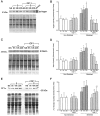
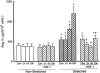
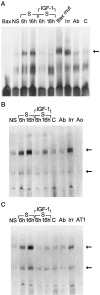
Insulin-like growth factor (IGF)-1 inhibits apoptosis, but its mechanism is unknown. Myocyte stretching activates p53 and p53-dependent genes, leading to the formation of angiotensin II (Ang II) and apoptosis. Therefore, this in vitro system was used to determine whether IGF-1 interfered with p53 function and the local renin-angiotensin system (RAS), decreasing stretch-induced cell death. A single dose of 200 ng/ml IGF-1 at the time of stretching decreased myocyte apoptosis 43% and 61% at 6 and 20 hours. Ang II concentration was reduced 52% at 20 hours. Additionally, p53 DNA binding to angiotensinogen (Aogen), AT1 receptor, and Bax was markedly down-regulated by IGF-1 via the induction of Mdm2 and the formation of Mdm2-p53 complexes. Concurrently, the quantity of p53, Aogen, renin, AT1 receptor, and Bax was reduced in stretched myocytes exposed to IGF-1. Conversely, Bcl-2 and the Bcl-2-to-Bax protein ratio increased. The effects of IGF-1 on cell death, Ang II synthesis, and Bax protein were the consequence of Mdm2-induced down-regulation of p53 function. In conclusion, the anti-apoptotic impact of IGF-1 on stretched myocytes was mediated by its capacity to depress p53 transcriptional activity, which limited Ang II formation and attenuated the susceptibility of myocytes to trigger their endogenous cell death pathway.
Figures


Similar articles
-
Inhibition of p53 function prevents renin-angiotensin system activation and stretch-mediated myocyte apoptosis.Am J Pathol. 2000 Sep;157(3):843-57. doi: 10.1016/S0002-9440(10)64598-1. Am J Pathol. 2000. PMID: 10980124 Free PMC article.
-
Overexpression of insulin-like growth factor-1 attenuates the myocyte renin-angiotensin system in transgenic mice.Circ Res. 1999 Apr 16;84(7):752-62. doi: 10.1161/01.res.84.7.752. Circ Res. 1999. PMID: 10205143
-
Stretch-mediated release of angiotensin II induces myocyte apoptosis by activating p53 that enhances the local renin-angiotensin system and decreases the Bcl-2-to-Bax protein ratio in the cell.J Clin Invest. 1998 Apr 1;101(7):1326-42. doi: 10.1172/JCI316. J Clin Invest. 1998. PMID: 9525975 Free PMC article.
-
Ischemic cardiomyopathy and the cellular renin-angiotensin system.J Heart Lung Transplant. 2000 Aug;19(8 Suppl):S1-11. doi: 10.1016/s1053-2498(99)00111-4. J Heart Lung Transplant. 2000. PMID: 11016481 Review.
-
Renin-angiotensin system, hypertrophy and gene expression in cardiac myocytes.J Mol Cell Cardiol. 1999 May;31(5):949-70. doi: 10.1006/jmcc.1999.0934. J Mol Cell Cardiol. 1999. PMID: 10336836 Review.
Cited by
-
Hdm2 is a ubiquitin ligase of Ku70-Akt promotes cell survival by inhibiting Hdm2-dependent Ku70 destabilization.Cell Death Differ. 2009 May;16(5):758-69. doi: 10.1038/cdd.2009.6. Epub 2009 Feb 27. Cell Death Differ. 2009. PMID: 19247369 Free PMC article.
-
Additive interaction between the renin-angiotensin system and lipid metabolism for cancer in type 2 diabetes.Diabetes. 2009 Jul;58(7):1518-25. doi: 10.2337/db09-0105. Epub 2009 Apr 28. Diabetes. 2009. PMID: 19401427 Free PMC article.
-
IGF-1 expression in infarcted myocardium and MGF E peptide actions in rat cardiomyocytes in vitro.Mol Med. 2009 May-Jun;15(5-6):127-35. doi: 10.2119/molmed.2009.00012. Epub 2009 Mar 6. Mol Med. 2009. PMID: 19295919 Free PMC article.
-
Growth hormone is permissive for neoplastic colon growth.Proc Natl Acad Sci U S A. 2016 Jun 7;113(23):E3250-9. doi: 10.1073/pnas.1600561113. Epub 2016 May 25. Proc Natl Acad Sci U S A. 2016. PMID: 27226307 Free PMC article.
-
Preoperative myocardial expression of E3 ubiquitin ligases in aortic stenosis patients undergoing valve replacement and their association to postoperative hypertrophy.PLoS One. 2020 Sep 18;15(9):e0237000. doi: 10.1371/journal.pone.0237000. eCollection 2020. PLoS One. 2020. PMID: 32946439 Free PMC article.
References
-
- Rodriguez-Tarduchy G, Collins MKL, Garcia I, Lopez-Rivas A: Insulin-like growth factor 1 inhibits apoptosis in IL-3 dependent hemopoietic cells. J Immunol 1992, 149:535-540 - PubMed
-
- Chun SY, Billig H, Tilly JL, Furuta I, Tsafriri A, Hsueh AJ: Gonadotropin suppression of apoptosis in cultured preovulatory follicles: mediatory role of endogenous insulin-like growth factor 1. Endocrinology 1994, 135:1845-1853 - PubMed
-
- Gluckman P, Klempt N, Guan J, Mallard C, Sirimanne E, Dragunow M, Klempt M, Singh K, Williams C, Nikolics K: A role for IGF-1 in the rescue of CNS neurons following hypoxic-ischemic injury. Biochem Biophys Res Commun 1992, 182:593-599 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

