The Gab1 PH domain is required for localization of Gab1 at sites of cell-cell contact and epithelial morphogenesis downstream from the met receptor tyrosine kinase
- PMID: 10022866
- PMCID: PMC83972
- DOI: 10.1128/MCB.19.3.1784
The Gab1 PH domain is required for localization of Gab1 at sites of cell-cell contact and epithelial morphogenesis downstream from the met receptor tyrosine kinase
Abstract
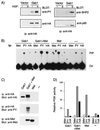
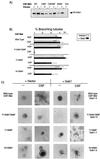
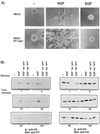
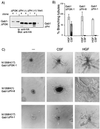
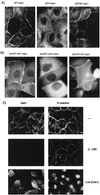
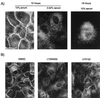
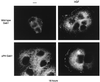
Stimulation of the hepatocyte growth factor (HGF) receptor tyrosine kinase, Met, induces mitogenesis, motility, invasion, and branching tubulogenesis of epithelial and endothelial cell lines in culture. We have previously shown that Gab1 is the major phosphorylated protein following stimulation of the Met receptor in epithelial cells that undergo a morphogenic program in response to HGF. Gab1 is a member of the family of IRS-1-like multisubstrate docking proteins and, like IRS-1, contains an amino-terminal pleckstrin homology domain, in addition to multiple tyrosine residues that are potential binding sites for proteins that contain SH2 or PTB domains. Following stimulation of epithelial cells with HGF, Gab1 associates with phosphatidylinositol 3-kinase and the tyrosine phosphatase SHP2. Met receptor mutants that are impaired in their association with Gab1 fail to induce branching tubulogenesis. Overexpression of Gab1 rescues the Met-dependent tubulogenic response in these cell lines. The ability of Gab1 to promote tubulogenesis is dependent on its pleckstrin homology domain. Whereas the wild-type Gab1 protein is localized to areas of cell-cell contact, a Gab1 protein lacking the pleckstrin homology domain is localized predominantly in the cytoplasm. Localization of Gab1 to areas of cell-cell contact is inhibited by LY294002, demonstrating that phosphatidylinositol 3-kinase activity is required. These data show that Gab1 is an important mediator of branching tubulogenesis downstream from the Met receptor and identify phosphatidylinositol 3-kinase and the Gab1 pleckstrin homology domain as crucial for subcellular localization of Gab1 and biological responses.
Figures


Similar articles
-
A conserved inositol phospholipid binding site within the pleckstrin homology domain of the Gab1 docking protein is required for epithelial morphogenesis.J Biol Chem. 1999 Oct 29;274(44):31719-26. doi: 10.1074/jbc.274.44.31719. J Biol Chem. 1999. PMID: 10531383
-
The multisubstrate adapter Gab1 regulates hepatocyte growth factor (scatter factor)-c-Met signaling for cell survival and DNA repair.Mol Cell Biol. 2001 Aug;21(15):4968-84. doi: 10.1128/MCB.21.15.4968-4984.2001. Mol Cell Biol. 2001. PMID: 11438654 Free PMC article.
-
Membrane targeting of Grb2-associated binder-1 (Gab1) scaffolding protein through Src myristoylation sequence substitutes for Gab1 pleckstrin homology domain and switches an epidermal growth factor response to an invasive morphogenic program.Mol Biol Cell. 2003 Apr;14(4):1691-708. doi: 10.1091/mbc.e02-06-0352. Mol Biol Cell. 2003. PMID: 12686619 Free PMC article.
-
The Role of GAB1 in Cancer.Cancers (Basel). 2023 Aug 20;15(16):4179. doi: 10.3390/cancers15164179. Cancers (Basel). 2023. PMID: 37627207 Free PMC article. Review.
-
MET meet adaptors: functional and structural implications in downstream signalling mediated by the Met receptor.Mol Cell Biochem. 2005 Aug;276(1-2):149-57. doi: 10.1007/s11010-005-3696-6. Mol Cell Biochem. 2005. PMID: 16132696 Review.
Cited by
-
Met receptor tyrosine kinase signals through a cortactin-Gab1 scaffold complex, to mediate invadopodia.J Cell Sci. 2012 Jun 15;125(Pt 12):2940-53. doi: 10.1242/jcs.100834. Epub 2012 Feb 24. J Cell Sci. 2012. PMID: 22366451 Free PMC article.
-
New role for Shc in activation of the phosphatidylinositol 3-kinase/Akt pathway.Mol Cell Biol. 2000 Oct;20(19):7109-20. doi: 10.1128/MCB.20.19.7109-7120.2000. Mol Cell Biol. 2000. PMID: 10982827 Free PMC article.
-
CD44v6+ Hepatocellular Carcinoma Cells Maintain Stemness Properties through Met/cJun/Nanog Signaling.Stem Cells Int. 2022 Nov 7;2022:5853707. doi: 10.1155/2022/5853707. eCollection 2022. Stem Cells Int. 2022. PMID: 36387747 Free PMC article.
-
Redundant roles for Met docking site tyrosines and the Gab1 pleckstrin homology domain in InlB-mediated entry of Listeria monocytogenes.Infect Immun. 2005 Apr;73(4):2061-74. doi: 10.1128/IAI.73.4.2061-2074.2005. Infect Immun. 2005. PMID: 15784547 Free PMC article.
-
Gab1 is required for cell cycle transition, cell proliferation, and transformation induced by an oncogenic met receptor.Mol Biol Cell. 2006 Sep;17(9):3717-28. doi: 10.1091/mbc.e06-03-0244. Epub 2006 Jun 14. Mol Biol Cell. 2006. PMID: 16775003 Free PMC article.
References
-
- Auger K R, Serunian L A, Soltoff S P, Libby P, Cantley L C. PDGF-dependent tyrosine phosphorylation stimulates production of novel polyphosphoinositides in intact cells. Cell. 1989;57:167–175. - PubMed
-
- Bardelli A, Longati P, Gramaglia D, Stella M C, Comoglio P M. Gab1 coupling to the HGF/Met receptor multifunctional docking site requires binding of Grb2 and correlates with the transforming potential. Oncogene. 1997;15:3103–3111. - PubMed
-
- Bellusci S, Moens G, Gaudino G, Comoglio P, Nakamura T, Thiery J P, Jouanneau J. Creation of an hepatocyte growth factor/scatter factor autocrine loop in carcinoma cells induces invasive properties associated with increased tumorigenicity. Oncogene. 1994;9:1091–1099. - PubMed
-
- Burks D J, Pons S, Towery H, Smith-Hall J, Myers M G, Jr, Yenush L, White M F. Heterologous pleckstrin homology domains do not couple IRS-1 to the insulin receptor. J Biol Chem. 1997;272:27716–27721. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
