Abstract
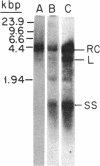
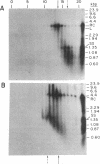
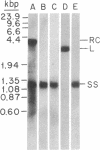
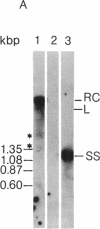
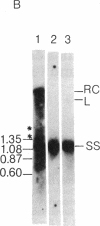
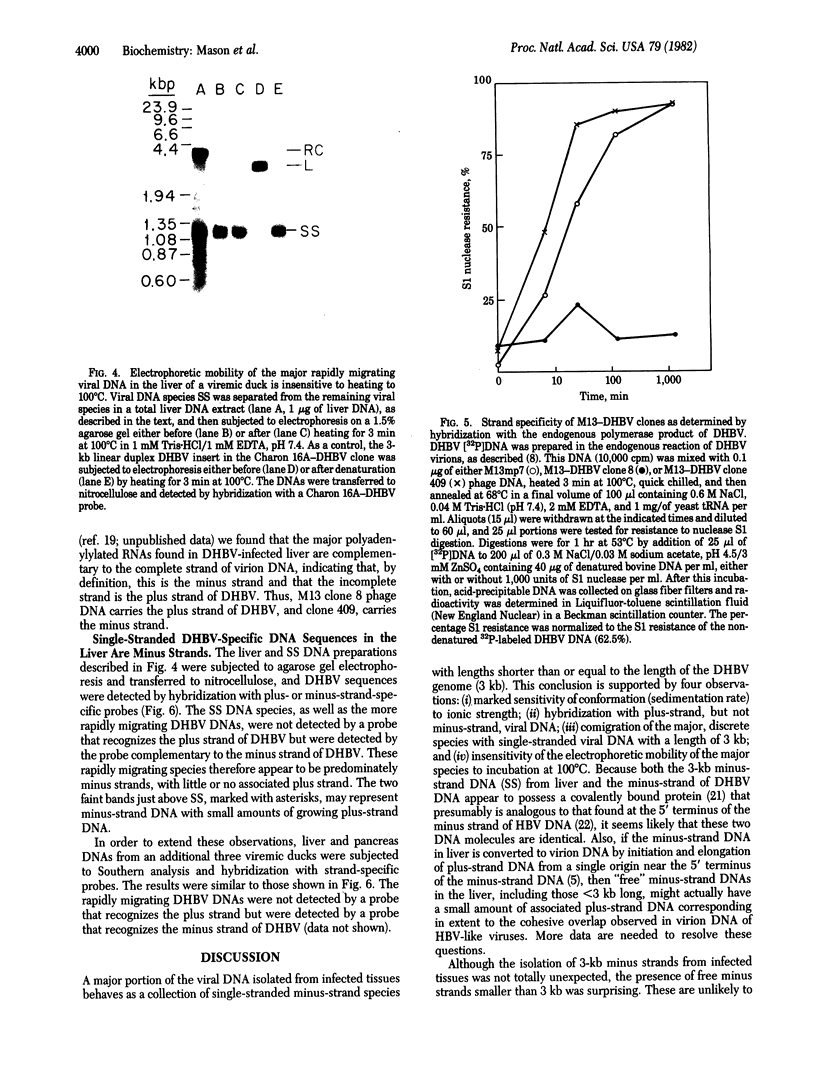
In order to study the replication of the DNA genome of duck hepatitis B virus, an avian virus related to human hepatitis B virus, we have characterized viral DNAs present in the livers of viremic ducks by agarose gel electrophoresis and the Southern blot procedure. In addition to relaxed circular DNA similar to virion DNA, livers contained a heterogeneous population of rapidly migrating species. The conformation of the rapidly migrating species was markedly sensitive to salt, suggesting that these species were largely single stranded. The largest major rapidly migrating species was shown to have an electrophoretic mobility that was insensitive to preheating of the DNA to 100 degrees C and was similar to that of denatured virus DNA 3 kilobases long, suggesting that this DNA was a single-stranded copy of the entire virus genome. Hybridization with strand-specific probes demonstrated that this 3-kilobase species, as well as more rapidly migrating DNAs, were predominantly minus strands.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Blattner F. R., Williams B. G., Blechl A. E., Denniston-Thompson K., Faber H. E., Furlong L., Grunwald D. J., Kiefer D. O., Moore D. D., Schumm J. W. Charon phages: safer derivatives of bacteriophage lambda for DNA cloning. Science. 1977 Apr 8;196(4286):161–169. doi: 10.1126/science.847462. [DOI] [PubMed] [Google Scholar]
- Brechot C., Hadchouel M., Scotto J., Degos F., Charnay P., Trepo C., Tiollais P. Detection of hepatitis B virus DNA in liver and serum: a direct appraisal of the chronic carrier state. Lancet. 1981 Oct 10;2(8250):765–768. doi: 10.1016/s0140-6736(81)90182-3. [DOI] [PubMed] [Google Scholar]
- Cummings I. W., Browne J. K., Salser W. A., Tyler G. V., Snyder R. L., Smolec J. M., Summers J. Isolation, characterization, and comparison of recombinant DNAs derived from genomes of human hepatitis B virus and woodchuck hepatitis virus. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1842–1846. doi: 10.1073/pnas.77.4.1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enquist L., Sternberg N. In vitro packaging of lambda Dam vectors and their use in cloning DNA fragments. Methods Enzymol. 1979;68:281–298. doi: 10.1016/0076-6879(79)68020-5. [DOI] [PubMed] [Google Scholar]
- Gerlich W. H., Robinson W. S. Hepatitis B virus contains protein attached to the 5' terminus of its complete DNA strand. Cell. 1980 Oct;21(3):801–809. doi: 10.1016/0092-8674(80)90443-2. [DOI] [PubMed] [Google Scholar]
- Hruska J. F., Clayton D. A., Rubenstein J. L., Robinson W. S. Structure of hepatitis B Dane particle DNA before and after the Dane particle DNA polymerase reaction. J Virol. 1977 Feb;21(2):666–672. doi: 10.1128/jvi.21.2.666-672.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. H., Grossman L. I. Electrophoresis of DNA in agarose gels. Optimizing separations of conformational isomers of double- and single-stranded DNAs. Biochemistry. 1977 Sep 20;16(19):4217–4225. doi: 10.1021/bi00638a014. [DOI] [PubMed] [Google Scholar]
- Marion P. L., Oshiro L. S., Regnery D. C., Scullard G. H., Robinson W. S. A virus in Beechey ground squirrels that is related to hepatitis B virus of humans. Proc Natl Acad Sci U S A. 1980 May;77(5):2941–2945. doi: 10.1073/pnas.77.5.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason W. S., Seal G., Summers J. Virus of Pekin ducks with structural and biological relatedness to human hepatitis B virus. J Virol. 1980 Dec;36(3):829–836. doi: 10.1128/jvi.36.3.829-836.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler F., Robinson W. S. Hepatitis B viral DNA molecules have cohesive ends. J Virol. 1979 Oct;32(1):226–233. doi: 10.1128/jvi.32.1.226-233.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sninsky J. J., Siddiqui A., Robinson W. S., Cohen S. N. Cloning and endonuclease mapping of the hepatitis B viral genome. Nature. 1979 May 24;279(5711):346–348. doi: 10.1038/279346a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Conformational changes of single-stranded DNA. J Mol Biol. 1969 Apr;41(2):189–197. doi: 10.1016/0022-2836(69)90384-2. [DOI] [PubMed] [Google Scholar]
- Summers J., O'Connell A., Millman I. Genome of hepatitis B virus: restriction enzyme cleavage and structure of DNA extracted from Dane particles. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4597–4601. doi: 10.1073/pnas.72.11.4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers J. Physical map of polyoma viral DNA fragments produced by cleavage with a restriction enzyme from Haemophilus aegyptius, endonuclease R-HaeIII. J Virol. 1975 Apr;15(4):946–953. doi: 10.1128/jvi.15.4.946-953.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers J., Smolec J. M., Snyder R. A virus similar to human hepatitis B virus associated with hepatitis and hepatoma in woodchucks. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4533–4537. doi: 10.1073/pnas.75.9.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]