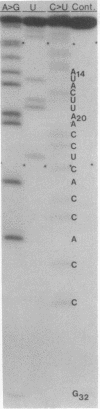
Abstract
Previous genetic and biochemical studies led to the identification of two large RNase T1-resistant oligonucleotides, designated the GIX+ and GIX- oligonucleotides, whose presence in the genomes of closely related murine leukemia viruses is mutually exclusive and predictive of two properties of the viral envelope glycoprotein gp70. Viruses harboring the GIX+ oligonucleotide induce expression of the gp70-associated antigen GIX and possess gp70s with more rapid electrophoretic mobility on sodium dodecyl sulfate/polyacrylamide gels than viruses that possess the GIX- oligonucleotide. The latter viruses fail to induce GIX on infected fibroblasts. The GIX+ and GIX- oligonucleotides lie in corresponding positions in the 3′ third of the oligonucleotide maps of their respective viruses. We have determined the nucleotide sequences of the GIX+ and GIX- oligonucleotides. The sequence of the GIX- oligonucleotide is U-A-U-C-U-C-A-A-C-C-A-C-C-A-U-A-C-U-U-A-A-C-C-U-C-A-C-C-A-C-[unk]-G, and the sequence of the GIX+ oligonucleotide is U-A-U-C-U-C-A-A-C-C-A-C-C-A-U-A-C-U-U-G. Thus, a single base change could result in the interconversion of the two oligonucleotides. Consideration of the amino acids specified by the two oligonucleotides suggests that this single base difference may result in the presence of an additional oligosaccharide chain in the gp70s of the GIX- viruses. Evidence supporting this prediction has been obtained by M. R. Rosner, J.-S. Tung, E. Fleissner, and P. W. Robbins (personal communication). It is entirely possible that the single nucleotide change that apparently results in a different electrophoretic mobility of the gp70s of the GIX+ and GIX- viruses is also responsible for the presence or absence of the GIX antigenic determinant; however, the validity of this possibility awaits further investigation.
Keywords: RNA nucleotide sequence determination, glycosylation
Full text
PDF
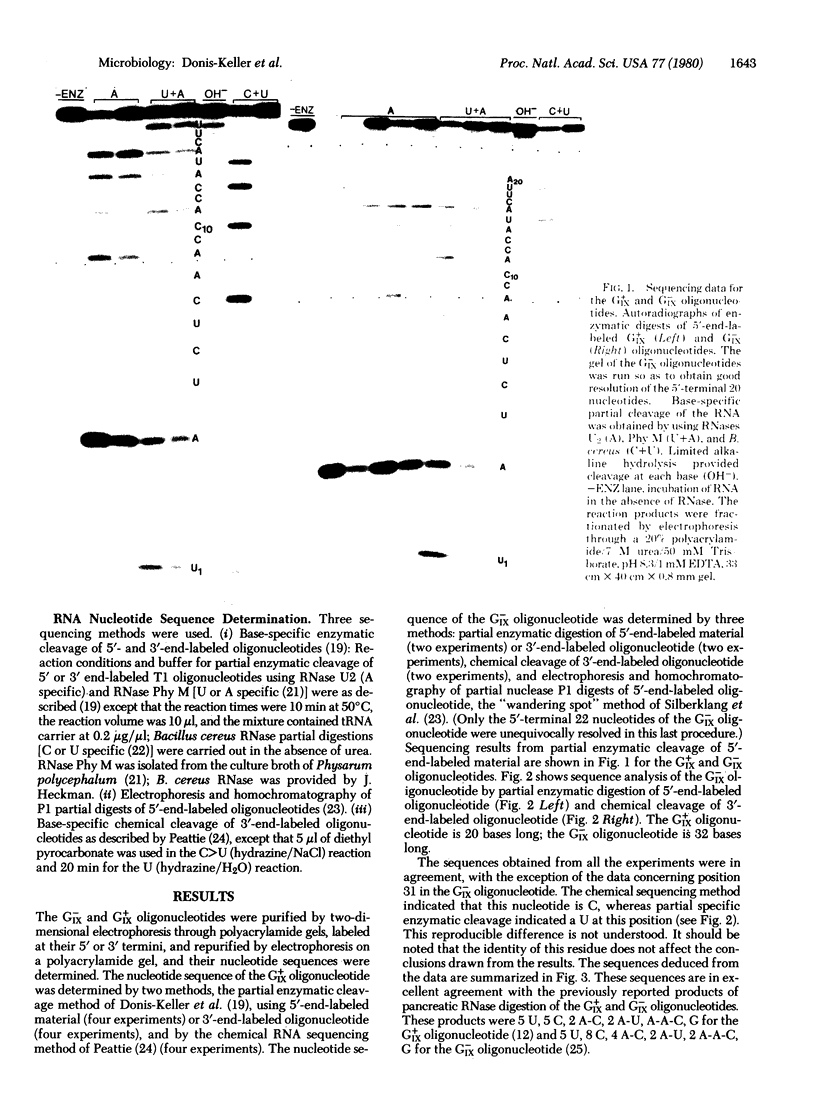
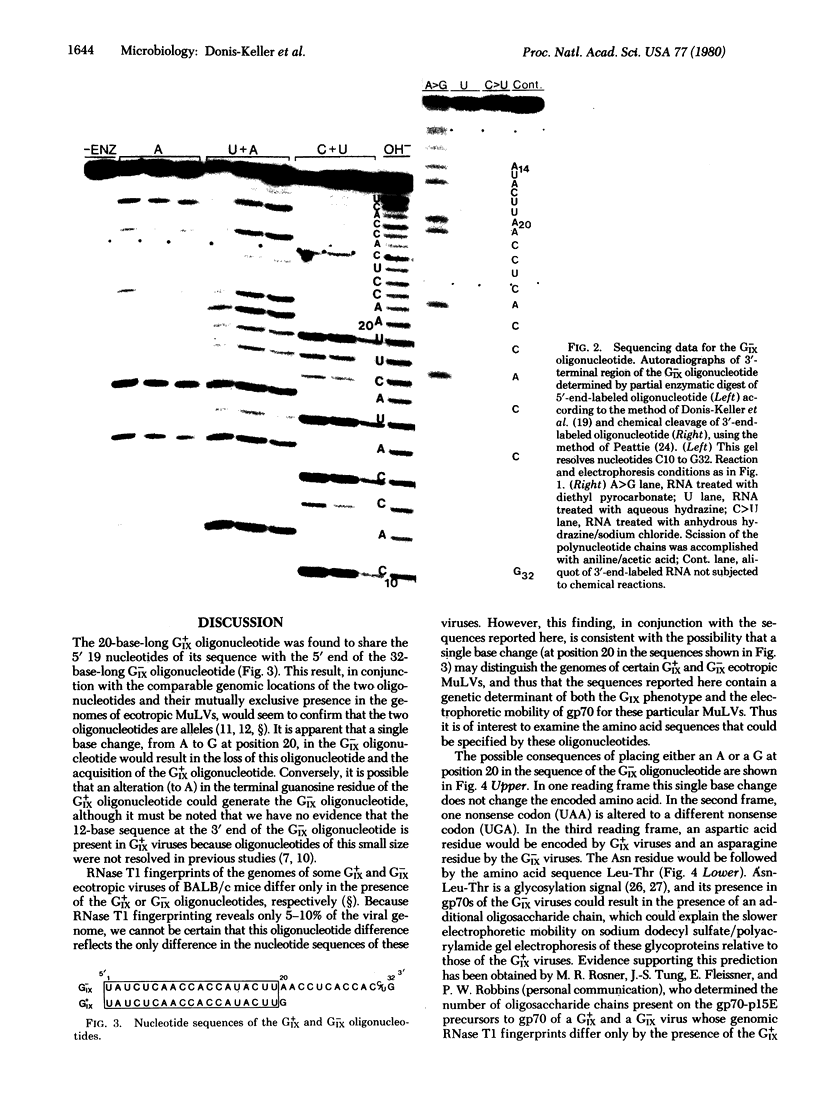

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England T. E., Uhlenbeck O. C. 3'-terminal labelling of RNA with T4 RNA ligase. Nature. 1978 Oct 12;275(5680):560–561. doi: 10.1038/275560a0. [DOI] [PubMed] [Google Scholar]
- Faller D. V., Hopkins N. RNase T1-resistant oligonucleotides of B-tropic murine leukemia virus from BALB/c and five of its NB-tropic derivatives. J Virol. 1977 Jul;23(1):188–195. doi: 10.1128/jvi.23.1.188-195.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faller D. V., Hopkins N. RNase T1-resistant oligonucleotides of an N- and a B-tropic murine leukemia virus of BALB/c: evidence for recombination between these viruses. J Virol. 1977 Nov;24(2):609–617. doi: 10.1128/jvi.24.2.609-617.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faller D. V., Hopkins N. T1 oligonucleotide maps of N-, B-, and B leads to NB-tropic murine leukemia viruses derived from BALB/c. J Virol. 1978 Apr;26(1):143–152. doi: 10.1128/jvi.26.1.143-152.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faller D. V., Hopkins N. T1 oligonucleotides that segregate with tropism and with properties of gp70 in recombinants between N- and B-tropic murine leukemia viruses. J Virol. 1978 Apr;26(1):153–158. doi: 10.1128/jvi.26.1.153-158.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faller D. V., Rommelaere J., Hopkins N. Large T1 oligonucleotides of Moloney leukemia virus missing in an env gene recombinant, HIX, are present on an intracellular 21S Moloney viral RNA species. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2964–2968. doi: 10.1073/pnas.75.6.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisby D. Oligonucleotide mapping of non-radioactive virus and messenger RNAs. Nucleic Acids Res. 1977 Sep;4(9):2975–2996. doi: 10.1093/nar/4.9.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P., Huebner R. J. Host-range restrictions of murine leukemia viruses in mouse embryo cell cultures. J Virol. 1970 Feb;5(2):221–225. doi: 10.1128/jvi.5.2.221-225.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins N., Schindler J., Gottlieb P. D. Evidence for recombination between N- and B-tropic murine leukemia viruses. J Virol. 1977 Mar;21(3):1074–1078. doi: 10.1128/jvi.21.3.1074-1078.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins N., Schindler J., Hynes R. Six-NB-tropic murine leukemia viruses derived from a B-tropic virus of BALB/c have altered p30. J Virol. 1977 Jan;21(1):309–318. doi: 10.1128/jvi.21.1.309-318.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockard R. E., Alzner-Deweerd B., Heckman J. E., MacGee J., Tabor M. W., RajBhandary U. L. Sequence analysis of 5'[32P] labeled mRNA and tRNA using polyacrylamide gel electrophoresis. Nucleic Acids Res. 1978 Jan;5(1):37–56. doi: 10.1093/nar/5.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall R. D. The nature and metabolism of the carbohydrate-peptide linkages of glycoproteins. Biochem Soc Symp. 1974;(40):17–26. [PubMed] [Google Scholar]
- O'Donnell P. V., Stockert E. Induction of GIX antigen and gross cell surface antigen after infection by ecotropic and xenotropic murine leukemia viruses in vitro. J Virol. 1976 Dec;20(3):545–554. doi: 10.1128/jvi.20.3.545-554.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata Y., Ikeda H., Stockert E., Boyse E. A. Relation of GIX antigen of thymocytes to envelope glycoprotein of murine leukemia virus. J Exp Med. 1975 Jan 1;141(1):188–197. doi: 10.1084/jem.141.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old L. J., Stockert E. Immunogenetics of cell surface antigens of mouse leukemia. Annu Rev Genet. 1977;11:127–160. doi: 10.1146/annurev.ge.11.120177.001015. [DOI] [PubMed] [Google Scholar]
- Peattie D. A. Direct chemical method for sequencing RNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1760–1764. doi: 10.1073/pnas.76.4.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pless D. D., Lennarz W. J. Enzymatic conversion of proteins to glycoproteins. Proc Natl Acad Sci U S A. 1977 Jan;74(1):134–138. doi: 10.1073/pnas.74.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommelaere J., Donis-Keller H., Hopkins N. RNA sequencing provides evidence for allelism of determinants of the N-, B- or NB-tropism of murine leukemia viruses. Cell. 1979 Jan;16(1):43–50. doi: 10.1016/0092-8674(79)90186-7. [DOI] [PubMed] [Google Scholar]
- Rommelaere J., Faller D. V., Hopkins N. RNase T1-resistant oligonucleotides of Akv-1 and Akv-2 type C viruses of AKR mice. J Virol. 1977 Nov;24(2):690–694. doi: 10.1128/jvi.24.2.690-694.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P. Studies of genetic transmission of murine leukemia virus by AKR mice. I. Crosses with Fv-1 n strains of mice. J Exp Med. 1972 Nov 1;136(5):1272–1285. doi: 10.1084/jem.136.5.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler J., Hynes R., Hopkins N. Evidence for recombination between N- and B-tropic murine leukemia viruses: analysis of three virion proteins by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. J Virol. 1977 Sep;23(3):700–700. doi: 10.1128/jvi.23.3.700-.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberklang M., Gillum A. M., RajBhandary U. L. The use of nuclease P1 in sequence analysis of end group labeled RNA. Nucleic Acids Res. 1977 Dec;4(12):4091–4108. doi: 10.1093/nar/4.12.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockert E., Old L. J., Boyse E. A. The G-IX system. A cell surface allo-antigen associated with murine leukemia virus; implications regarding chromosomal integration of the viral genome. J Exp Med. 1971 Jun 1;133(6):1334–1355. doi: 10.1084/jem.133.6.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung J. S., Vitetta E. S., Fleissner E., Boyse E. A. Biochemical evidence linking the GIX thymocyte surface antigen to the gp69/71 envelope glycoprotein of murine leukemia virus. J Exp Med. 1975 Jan 1;141(1):198–205. doi: 10.1084/jem.141.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wachter R., Fiers W. Preparative two-dimensional polyacrylamide gel electrophoresis of 32 P-labeled RNA. Anal Biochem. 1972 Sep;49(1):184–197. doi: 10.1016/0003-2697(72)90257-6. [DOI] [PubMed] [Google Scholar]