Abstract
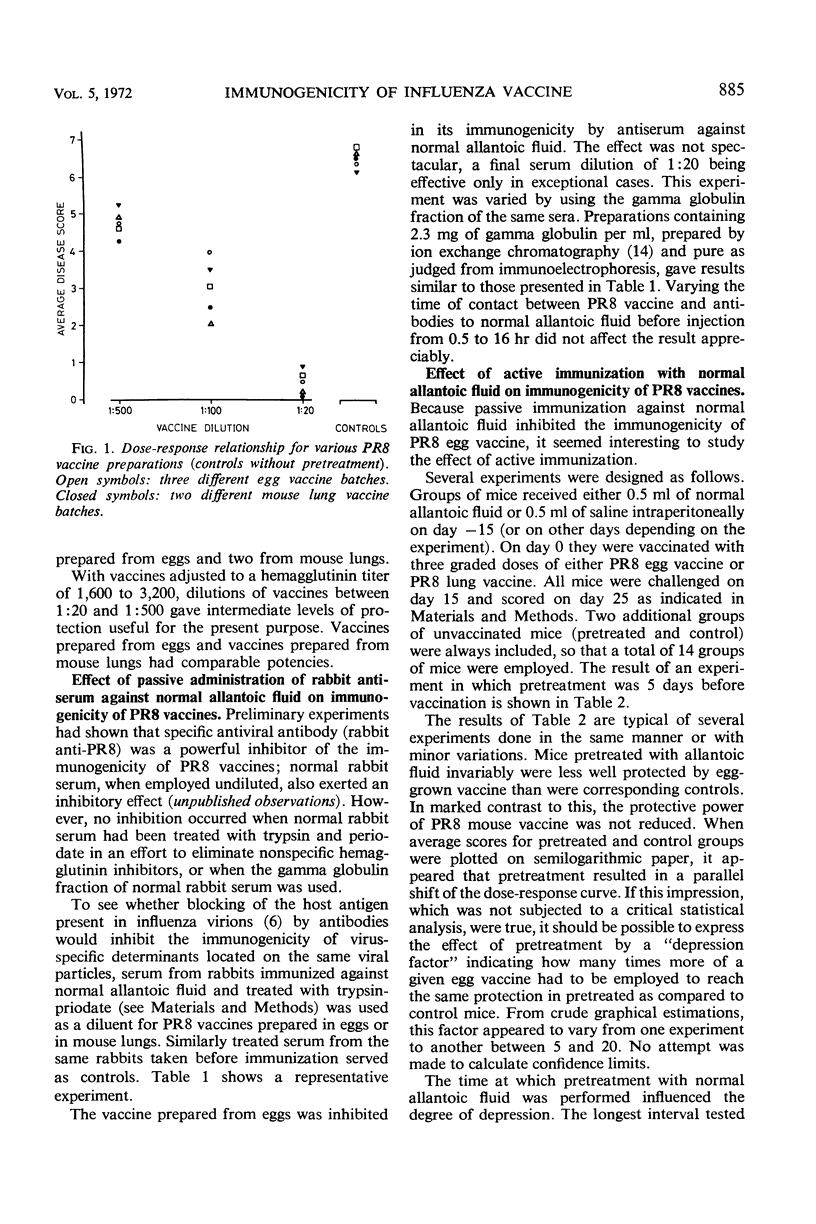
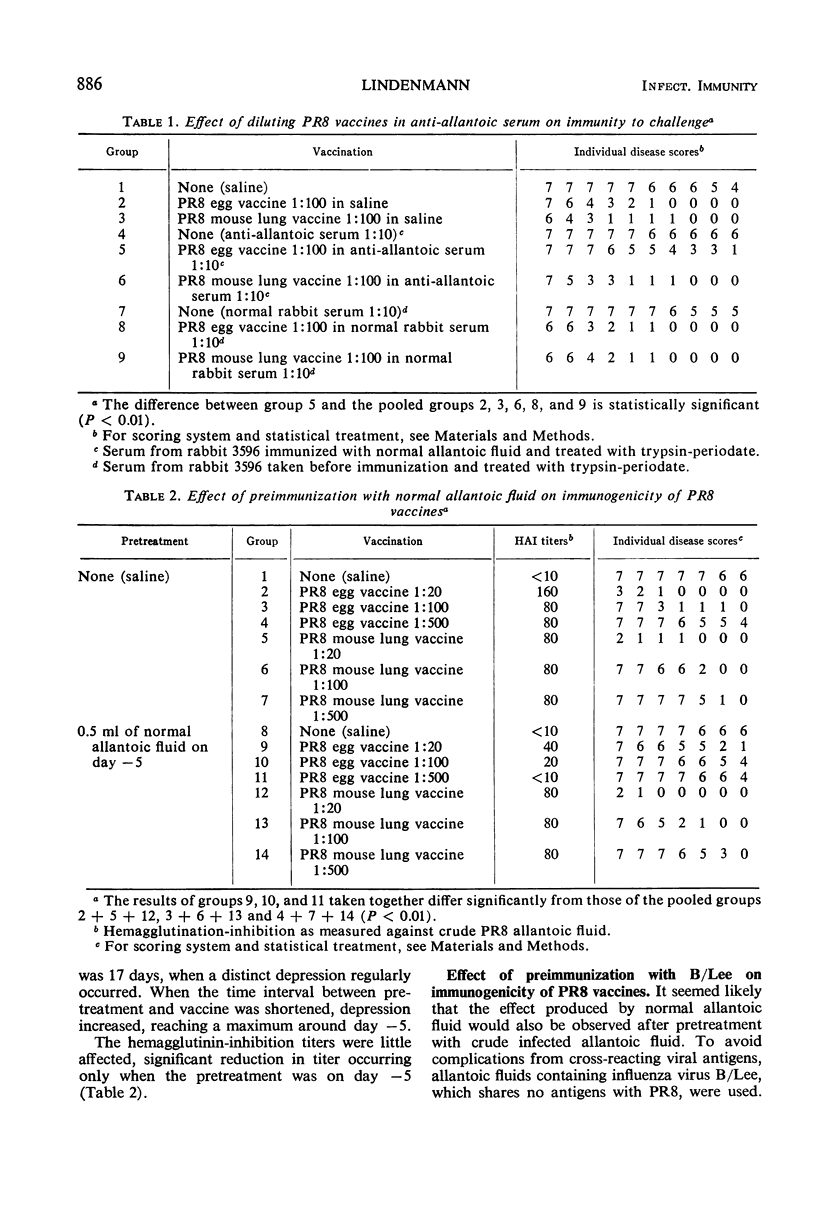
Rabbit antibody directed against normal allantoic fluid reduced the protective power of influenza vaccine prepared from egg-grown PR8 virus. A similar vaccine prepared from mouse lung virus was not inhibited. Preimmunization of mice with normal allantoic fluid or with egg-grown influenza B/Lee vaccine (crude or purified) inhibited the protective power of PR8 egg vaccine but did not affect a similar lung vaccine.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DE SAMPAIO A. A. C., ISAACS A. The action of trypsin on normal serum inhibitors of influenza virus agglutination. Br J Exp Pathol. 1953 Apr;34(2):152–158. [PMC free article] [PubMed] [Google Scholar]
- Eickhoff T. C. Immunization against influenza: rationale and recommendations. J Infect Dis. 1971 Apr;123(4):446–454. doi: 10.1093/infdis/123.4.446. [DOI] [PubMed] [Google Scholar]
- HARBOE A. The influenza virus haemagglutinnation inhibition by antibody to host material. Acta Pathol Microbiol Scand. 1963;57:317–330. doi: 10.1111/j.1699-0463.1963.tb05101.x. [DOI] [PubMed] [Google Scholar]
- Henney C. S. Studies on the mechanism of suppression of the immune response by antibody. Int Arch Allergy Appl Immunol. 1971;40(3):372–381. doi: 10.1159/000230420. [DOI] [PubMed] [Google Scholar]
- Henry C., Jerne N. K. Competition of 19S and 7S antigen receptors in the regulation of the primary immune response. J Exp Med. 1968 Jul 1;128(1):133–152. doi: 10.1084/jem.128.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz D. H., Paul W. E., Goidl E. A., Benacerraf B. Carrier function in anti-hapten immune responses. I. Enhancement of primary and secondary anti-hapten antibody responses by carrier preimmunization. J Exp Med. 1970 Aug 1;132(2):261–282. doi: 10.1084/jem.132.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye H. S., Dowdle W. R., McQueen J. L. Studies on inactivated influenza vaccines. I. The effect of dosage on antibody response and protection against homotypic and heterotypic influenza virus challenge in mice. Am J Epidemiol. 1969 Aug;90(2):162–169. doi: 10.1093/oxfordjournals.aje.a121060. [DOI] [PubMed] [Google Scholar]
- LEVY H. B., SOBER H. A. A simple chromatographic method for preparation of gamma globulin. Proc Soc Exp Biol Med. 1960 Jan;103:250–252. doi: 10.3181/00379727-103-25476. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Webster R. G. The structure of influenza viruses. IV. Chemical studies of the host antigen. Virology. 1966 Sep;30(1):104–115. doi: 10.1016/s0042-6822(66)81014-0. [DOI] [PubMed] [Google Scholar]
- Lindenmann J. Cross-priming and cross-inhibition by antibody in the influenza virus--host antigen system. Proc R Soc Lond B Biol Sci. 1971 Jan 12;176(1045):419–423. doi: 10.1098/rspb.1971.0004. [DOI] [PubMed] [Google Scholar]
- Lindenmann J., Klein P. A. Viral oncolysis: increased immunogenicity of host cell antigen associated with influenza virus. J Exp Med. 1967 Jul 1;126(1):93–108. doi: 10.1084/jem.126.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum S. C., Mostow S. R., Dowdle W. R., Coleman M. T., Kaye H. S. Studies with inactivated influenza vaccines purified by zonal centrifugation. 2. Efficacy. Bull World Health Organ. 1969;41(3):531–535. [PMC free article] [PubMed] [Google Scholar]


