Abstract
Background
Open surgical debridement was the main treatment option for infected pancreatic necrosis (IPN). However, it was associated with significant trauma, leading to a higher mortality rate. With the development of minimally invasive surgery, the step-up treatment principle centered around minimally invasive intervention, significantly reducing the incidence of complications and mortality rates among IPN patients. However, few studies have reported the efficacy of laparoscopic retroperitoneal pancreatic necrosectomy (LRPN), a new minimally invasive debridement technique, in IPN patients with duodenal fistula (DF)—a severe complication of IPN. Therefore, we analyzed the effectiveness and safety of LRPN for treating IPN with DF and discussed the impact of DF on patient prognosis.
Methods
We retrospectively examined patients diagnosed with IPN between 2018 and 2023. The patients were divided into two groups based on the presence or absence of DF. Clinical characteristics, treatment strategies, clinical outcomes, and follow-up information were analyzed. A 1:1 propensity score-matching (PSM) method was used to assess differences in outcome indicators more accurately.
Results
A total of 197 patients were examined. After PSM, no significant differences were observed between the two groups in in-hospital mortality rate, incidence of single organ failure, rate of postoperative severe complications (Clavien–Dindo Classification ≥ 3), and intensive care unit stay (P > 0.05). However, the incidence of multiorgan failure, gastrointestinal bleeding, number of percutaneous catheter drainage (PCD) procedures, surgery cases, hospital stay, and hospitalization costs were higher in the DF group (P < 0.05). Of these patients, 71.6% (n = 141) were treated with PCD + LRPN, with a conversion rate of 6.38% to open surgery. A higher proportion of patients in the non-DF group showed improved clinical outcomes solely with PCD (22.6% vs. 2.4%, P < 0.05), whereas a higher proportion of patients in the DF group underwent PCD + LRPN (88.1% vs. 67.1%, P < 0.05). Both groups showed a significant reduction in the Sequential Organ Failure Assessment score 72 h postoperatively.
Conclusions
For patients with IPN and DF, the LRPN-centered step-up strategy was safe and effective. DF prolongs hospital stay and increases hospitalization costs for patients.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12876-024-03539-7.
Keywords: Duodenal fistula, Infected necrotizing pancreatitis, Laparoscopic retroperitoneal pancreatic necrosectomy, Propensity score matching
Background
Acute pancreatitis is a common digestive system disease that is attracting attention owing to its significant incidence and mortality rates. The incidence of pancreatitis has increased worldwide in recent years [1]. Approximately 80% of pancreatitis cases are interstitial edematous pancreatitis, which is self-limiting and typically improves within approximately 1 week. However, about 20% of pancreatitis cases will progress to necrotizing pancreatitis, with a mortality rate as high as 20% [2, 3]. The high mortality rate of necrotizing pancreatitis is closely related to the local and systemic complications caused by pancreatic/peri-pancreatic tissue necrosis [4]. When pancreatic necrosis is complicated by an infection, especially when combined with gastrointestinal fistulas, the risk of death increases significantly. Gastrointestinal fistula is a severe complication of infected necrotizing pancreatitis, with duodenal fistula (DF) being the most common, second only to colonic fistulas [5]. The specific mechanism of DF formation is not yet clear; however, because of the close anatomical relationship between the duodenum and pancreas, along with factors such as corrosive effects of pancreatic enzymes and necrotic substances around the pancreas, infection with necrotizing pancreatitis is more likely to lead to DF [6]. DF can exacerbate systemic infections, abdominal bleeding, malnutrition, and other local and systemic complications, thereby affecting the treatment process and prognosis [7, 8].
More than 10 years ago, open surgical debridement was the main treatment option for infected pancreatic necrosis (IPN). However, it was associated with significant trauma, leading to a higher mortality rate. With the development of minimally invasive surgery and promotion of damage control, the step-up treatment principle centered around minimally invasive intervention and debridement gradually became the mainstream approach for IPN treatment, significantly reducing the incidence of complications and mortality rates among IPN patients [9, 10]. However, few studies have reported the efficacy of laparoscopic retroperitoneal pancreatic necrosectomy (LRPN), a new minimally invasive debridement technique, in IPN patients with DF. Therefore, this study designed a retrospective cohort study primarily focused on the effectiveness and safety of LRPN for treating IPN with concurrent DF and discussed the impact of DF on prognosis.
Methods
Research design
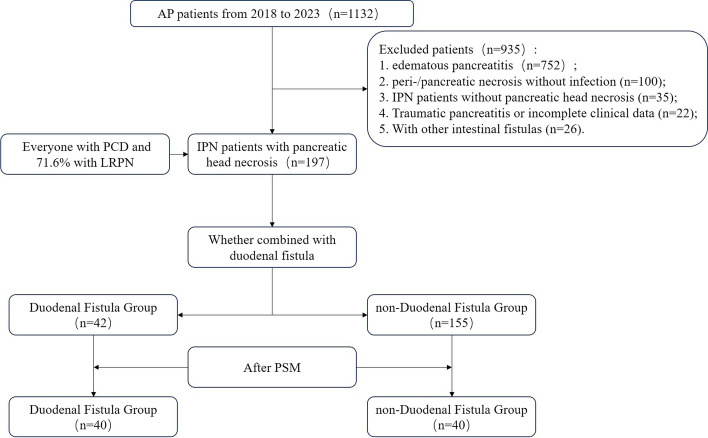
This study retrospectively analyzed the clinical and follow-up data of patients diagnosed with IPN complicated by pancreatic head necrosis who were admitted to the Severe Pancreatitis Center of Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, between January 2018 and October 2023. Patients entering the final cohort were divided into DF and non-DF groups based on the presence or absence of DF. The study adhered to the STROBE guidelines for observational studies [11] and the principles of the Helsinki Declaration (2013) [12] and was approved by the Sir Run Run Shaw Hospital Ethics Committee (No.2024–0214). Written informed consent was obtained from all patients. The clinical data of all enrolled patients were obtained from the clinical information system and analyzed anonymously. A flowchart of the study is shown in Fig. 1.
Fig. 1.
The study flow chart
Inclusion and exclusion criteria
According to the diagnostic criteria for pancreatitis [13], all patients diagnosed with necrotic pancreatitis and who had a confirmed infection of peri/pancreatic tissue necrosis on enhanced computed tomography (CT), magnetic resonance imaging (MRI), or culture of pancreatic necrosis aspirate were included in the final cohort if they do not meet the exclusion criteria.
The exclusion criteria were as follows: 1) edematous pancreatitis or pancreatic/peri-pancreatic necrosis without infection, 2) traumatic pancreatitis requiring emergency surgery, 3) without concurrent pancreatic head necrosis, 4) incomplete clinical data or follow-up information, and 5) In addition to duodenal fistula, other intestinal fistulas such as colon fistula are also present.
Diagnostic criteria of DF
A diagnosis of DF was considered definite if one of the following criteria was met: 1) During surgery, it was confirmed that there was a duodenal fistula; 2) endoscopic examination revealed the formation of duodenal fistula; and 3) upper gastrointestinal or sinus tract radiography showed extravasation of the contrast agent from the duodenum.
Clinical treatment strategy
Upon admission, all patients with necrotizing pancreatitis underwent a comprehensive assessment by a multidisciplinary team according to the pancreatitis diagnosis and treatment guidelines [14]. Standard treatments such as goal-directed fluid resuscitation, inhibition of pancreatic enzyme secretion and activity, pain management, and nutritional support were implemented. Treatment strategies, including organ function support therapy, were actively adjusted according to the patient’s clinical condition. If the conservative treatment was ineffective, a step-up treatment strategy was initiated [15].
Step-up treatment strategy centered around laparoscopic retroperitoneal pancreatic necrosectomy
In the first step, percutaneous catheter drainage (PCD) was performed under ultrasound or CT guidance to drain the accumulation of infected fluid around the pancreas. Regular abdominal CT was conducted, and PCD was repeated and gradually enlarged based on the accumulation of intra-abdominal fluid to improve drainage efficacy. The drained pus was cultured for bacteria, and antibiotic sensitivity testing was performed. Antibiotics were administered based on sensitivity results to enhance anti-infective treatment. If drainage was effective and the intra-abdominal infection gradually improved, further surgical debridement was not necessary.
If drainage was ineffective, laparoscopic-assisted retroperitoneal drainage of the pancreatic necrotic aggregates was performed. The patient was positioned in the left lateral decubitus position at an angle of 80–90°. A 2 cm incision was made along the posterior axillary line, between the right costal margin and the right iliac bone, and the retroperitoneal space was entered under direct vision. The pre-placed PCD tube served as a guide during the procedure. Two additional incisions, measuring 10 mm and 5 mm, were made on either side of the initial incision, and the abdominal cavity was insufflated to a pressure of 10 mmHg. Necrotic tissue was cleared under laparoscopic guidance with the assistance of oval forceps, if necessary, to improve debridement efficiency. For patients suggested of having DF, infected necrotic aggregates around the duodenum were cleared, and a double-sleeve tube was placed around the fistula site for continuous postoperative irrigation. In cases of uncontrollable intraoperative bleeding, open surgery was performed for hemostasis and debridement. Following debridement, double-sleeve tubes were placed in the retroperitoneal cavity for continuous negative-pressure irrigation. For patients with concurrent DFs, an enteric nutrition tube was placed across the fistula to avoid oral intake, and intestinal and parenteral nutrition were adjusted as needed.
Observation and follow-up indicators
The observation and follow-up indicators included causes, diagnostic methods, and treatment modalities of DF; in-hospital mortality rate; incidence of persistent organ failure or new-onset organ failure; incidence of single and multiple organ failure; duration of respiratory failure; rate of complications with Clavien–Dindo Classification (CDC) ≥ 3; incidence of intra-abdominal and gastrointestinal bleeding; number of PCD and surgical procedures; incidence of systemic infections such as blood and respiratory system infections; trend of perioperative Sequential Organ Failure Assessment (SOFA) score changes; length of intensive care unit stay; total length of hospital stay; and hospitalization costs. Discharged patients were followed up for at least 6 months, with a follow-up deadline of April 30, 2024. For patients who were not followed up in outpatient clinics, follow-up information was obtained via telephone interviews. The clinical observation parameters and definitions used in this study are listed in Table S1.
Statistical analysis
Continuous variables that did not conform to a normal distribution based on normality tests are presented as median (interquartile range), and differences between the two groups were compared using the Mann–Whitney U test. Categorical variables are presented as number (percentages), and differences between the two groups were compared using the chi-square test or Fisher’s exact test. Statistical analyses were performed using the Statistical Package for the Social Sciences version 23.0, (IBM Corp., Armonk, NY) and GraphPad Prism 8.0 (GraphPad Software, La Jolla, CA). To balance baseline characteristics and the influence of inconsistent clinical features at admission on the evaluation of clinical outcomes between patients with and without DF, researchers used the nearest neighbor propensity score matching (PSM) method for 1:1 matching with a matching tolerance of 0.03. Survival analysis was conducted using the Kaplan–Meier method, and differences were compared using the log-rank test. Statistical significance was set at P < 0.05.
Results
Demographic
From January 2018 to October 2023, 1132 cases with pancreatitis were treated at our center, with an incidence rate of necrotizing pancreatitis of 31.5% (n = 357); IPN, 22.7% (n = 257); and DF, 4.4% (n = 50). On the basis of the inclusion and exclusion criteria, 197 patients with IPN and concurrent pancreatic head necrosis were included (Fig. 1).
Propensity score matching analysis
Matching options included sex; age; body mass index; etiology of pancreatitis; American Society of Anesthesiologists classification; Charlson Comorbidity Index; Computer Tomography Severity Index; grading of the extent of pancreatic necrosis; and levels of hemoglobin, serum albumin, total bilirubin, creatinine, and plasma procalcitonin at admission. After matching, baseline data and clinical indicators at admission were comparable between the two groups (P > 0.05; Table 1).
Table 1.
Baseline Data of the Two Groups Before and After PSM
| Characteristics | Before PSM | P-value | After PSM | P-value | |||
|---|---|---|---|---|---|---|---|
| Duodenal Fistula Group(n = 42) | non-Duodenal Fistula Group(n = 155) | Duodenal Fistula Group(n = 40) | non-Duodenal Fistula Group(n = 40) | ||||
|
Age, year [n (%)] |
< 60 | 31(73.8) | 113(72.9) | 0.906 | 30 (75.0) | 34 (85.0) | 0.264 |
| ≥ 60 | 11 (26.2) | 42 (27.1) | 10 (25.0) | 6 (15.0) | |||
|
Gender [n (%)] |
male | 23 (54.8) | 110 (71.0) | 0.047* | 23 (57.5) | 23 (57.5) | 1.00 |
| female | 19 (45.2) | 45 (29.0) | 17 (42.5) | 17 (42.5) | |||
|
BMI, Kg/m2 [n (%)] |
< 25 | 25 (59.5) | 77 (49.7) | 0.257 | 23 (57.5) | 24 (60.0) | 0.82 |
| ≥ 25 | 17 (40.5) | 78 (50.3) | 17 (42.5) | 16 (40.0) | |||
|
Etiology [n (%)] |
Biliary | 18 (42.9) | 57 (36.8) | 0.758 | 18 (45.0) | 16 (40.0) | 0.746 |
| HTG | 14 (33.3) | 55 (35.5) | 13 (32.5) | 12 (30.0) | |||
| Others | 10 (23.8) | 43 (27.7) | 9 (22.5) | 12 (30.0) | |||
|
ASA score [n (%)] |
2 | 15 (35.7) | 53 (34.2) | 0.086 | 15 (37.5) | 20 (50.0) | 0.148 |
| 3 | 26 (61.9) | 80 (51.6) | 24 (60.0) | 16 (40.0) | |||
| 4 | 1 (2.4) | 22 (14.2) | 1 (2.5) | 4 (10.0) | |||
|
CCI Score [n (%)] |
< 3 | 26 (61.9) | 106 (63.4) | 0.428 | 26 (65.0) | 31 (77.5) | 0.217 |
| ≥ 3 | 16 (38.1) | 49 (36.6) | 14 (35.0) | 9 (22.5) | |||
|
CTSI Scores [n (%)] |
< 8 | 9 (21.4) | 54 (34.8) | 0.098 | 9 (22.5) | 13 (32.5) | 0.317 |
| ≥ 8 | 33 (78.6) | 101 (65.2) | 31 (77.5) | 27 (67.5) | |||
|
Extent of necrosis [n (%)] |
< 30% | 9 (21.4) | 47 (30.3) | 0.024* | 9 (22.5) | 10 (25.0) | 0.498 |
| 30–50% | 13 (31.0) | 68 (43.9) | 13 (32.5) | 17 (42.5) | |||
| ≥ 50 | 20 (47.6) | 40 (25.8) | 18 (45.0) | 13 (32.5) | |||
|
Hb (g/L) [n (%)] |
< 90 | 22 (52.4) | 71 (45.8) | 0.449 | 20 (50.0) | 23 (57.5) | 0.50 |
| ≥ 90 | 20 (47.6) | 84 (54.2) | 20 (50.0) | 17 (42.5) | |||
|
Alb (g/L) [n (%)] |
< 30 | 27 (62.3) | 98 (63.2) | 0.899 | 26 (65.0) | 26 (65.0) | 1.00 |
| ≥ 30 | 15 (37.7) | 57 (36.8) | 14 (35.0) | 14 (35.0) | |||
|
TBil (umol/L) [n (%)] |
< 33 | 30 (71.4) | 112 (72.3) | 0.915 | 29 (72.5) | 30 (75.0) | 0.799 |
| ≥ 33 | 12 (28.6) | 43 (27.7) | 11 (27.5) | 10 (25.0) | |||
|
Cr (umol/L) [n (%)] |
< 110 | 31 (73.8) | 102 (65.8) | 0.326 | 30 (75.0) | 31 (77.5) | 0.739 |
| ≥ 110 | 11 (26.2) | 53 (34.2) | 10 (25.0) | 9 (22.5) | |||
|
PCT (ng/mL) [n (%)] |
< 0.5 | 14 (33.3) | 54 (34.8) | 0.856 | 14 (35.0) | 15 (37.5) | 0.816 |
| ≥ 0.5 | 28 (66.7) | 101 (65.2) | 26 (65.0) | 25 (62.5) | |||
*P < 0.05
Abbreviations PSM Propensity Score Matching, BMI Body mass index, HTG Hypertriglyceridemia, ASA American Society of Anesthesiologists, CCI Charlson Comorbidity Index, CTSI Computer Tomography Severity Index, Hb Hemoglobin, Alb Albumin, TBil Total Bilirubin, Cr Creatinine, PCT Procalcitonin
After PSM, clinical outcome analysis revealed no significant differences between the two groups in in-hospital mortality rate, incidence of respiratory and renal failure, occurrence of single organ failure, incidence of systemic infections such as blood and respiratory system infections, intra-abdominal bleeding rate, rate of postoperative complications with CDC ≥ 3, and length of intensive care unit stay (P > 0.05). However, the incidence of persistent cardiac failure, multiple organ failure, and gastrointestinal bleeding; surgical debridement cases, PCD and surgical debridement cases, total length of hospital stay, and total hospitalization costs were significantly higher in the DF group than in the non-DF group (P < 0.05; Table 2).
Table 2.
Comparison of clinical outcomes between the two groups before and after PSM
| Outcome indicators | Before PSM | OR (95%CI) /Z | P-value | After PSM | OR (95%CI) /Z | P-value | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Duodenal Fistula Group(n = 42) | non-Duodenal Fistula Group (n = 155) | Duodenal Fistula Group(n = 40) | non-Duodenal Fistula Group (n = 40) | |||||||
| In-hospital mortality [n (%)] | 6 (14.3) | 15 (9.7) |
1.556 (0.564, 4.293) |
0.402 | 4 (10.0) | 2 (5.0) |
2.111 (0.364, 12.24) |
0.675 | ||
| Persistent organ failure [n (%)] | ||||||||||
| Respiratory failure | 23 (54.8) | 56 (36.1) |
2.140 (1.073, 4.268) |
0.029* | 22 (55.0) | 14 (35.0) |
2.270 (0.923, 5.583) |
0.072 | ||
| Circulatory failure | 14 (33.3) | 30 (19.4) |
2.083 (0.979, 4.434) |
0.054 | 13 (32.5) | 3 (7.5) |
5.938 (1.540, 22.903) |
0.005* | ||
| Renal failure | 4 (9.5) | 20 (12.9) |
1.125 (0.421, 3.008) |
0.814 | 3 (7.5) | 3 (7.5) |
1.0 (0.189, 5.280) |
1.00 | ||
| Single organ failure | 12 (28.6) | 33 (21.3) |
1.479 (0.683, 3.20) |
0.319 | 11 (27.5) | 11 (27.5) |
1.00 (0.375, 2.669) |
1.00 | ||
| Multiple organ failure | 13 (31.0) | 31 (20.0) |
1.793 (0.863, 3.847) |
0.131 | 12 (30.0) | 4 (10.0) |
3.857 (1.122, 13.258) |
0.025* | ||
| Duration of respiratory failure, d [Median (IQR)] | 3 (0–14.0) | 3 (0–13.0) | -0.074 | 0.941 | 1.5 (0–12.75) | 1.5 (0–7.5) | -0.555 | 0.579 | ||
| Major infectious complications [n (%)] | ||||||||||
| Blood infection | 9 (21.4) | 29 (18.7) |
1.185 (0.551, 2.746) |
0.692 | 8 (20.0) | 4 (10.0) |
2.250 (0.619, 8.184) |
0.21 | ||
| Respiratory infection | 24 (57.1) | 78 (50.3) |
1.316 (0.662, 2.618) |
0.433 | 22 (55.0) | 17 (42.5) |
1.654 (0.683, 4.002) |
0.263 | ||
| Abdominal infection | 9 (21.4) | 15 (9.7) |
2.545 (1.025, 6.319) |
0.039* | 9 (22.5) | 5 (12.5) |
2.032 (0.615, 6.716) |
0.239 | ||
| Urinary tract infection | 14 (33.3) | 36 (23.2) |
1.653 (0.787, 3.471) |
0.182 | 13 (32.5) | 6 (15.0) |
2.728 (0.916, 8.126) |
0.066 | ||
| Abdominal bleeding [n (%)] | 15 (35.7) | 39 (22.6) |
1.652 (0.798, 3.422) |
0.174 | 15 (37.5) | 8 (20.0) |
2.40 (0.879, 6.556) |
0.084 | ||
|
Gastrointestinal bleeding [n (%)] |
9 (21.4) | 4 (2.6) |
10.295 (2.990, 35.454) |
0.00* | 9 (22.5) | 1 (2.5) |
11.323 (1.360, 94.248) |
0.007* | ||
| CDC ≥ 3 [n (%)] | 28 (66.7) | 86 (55.5) |
1.605 (0.785, 3.282) |
0.193 | 28 (70.0) | 20 (50.0) |
2.333 (0.932, 5.839) |
0.068 | ||
|
Number of surgical patients [n (%)] |
40 (95.2) | 119 (76.8) |
6.050 (1.394, 26.269) |
0.007* | 38 (95.0) | 30 (75.0) |
6.333 (1.289, 31.115) |
0.012* | ||
|
Number of surgeries [Median (IQR)] |
1 (1–2) | 1 (1–1) | -3.432 | 0.001* | 1 (1–2) | 1 (0.25–1) | -3.41 | 0.001* | ||
| PCD times [Median (IQR)] | 2 (1–3) | 1 (1–2) | -3.086 | 0.002* | 2 (1–3) | 1 (1–2) | -2.016 | 0.044* | ||
| ICU stays, d [Median (IQR)] | 14 (3.75–38.0) | 12 (3.0–30.0) | -0.855 | 0.392 | 12 (3.25–33.0) | 9.5 (2.0–22.75) | -1.474 | 0.14 | ||
|
Hospital stays, d [Median (IQR)] |
89 (47.8–118.5) |
55 (31.0–81.0) |
-3.747 | 0.00* |
90 (55.5–123.5) |
48.5 (35.25–79.0) |
-3.205 | 0.001* | ||
|
Hospitalization costs (thousand yuan) [Median (IQR)] |
403.6 (210.4–663.7) |
252.0 (121.6–412.4) |
-2.828 | 0.005* |
394.28 (197.04–666.0) |
206.78 (108.77–654.12) |
-3.07 | 0.002* | ||
*P < 0.05
Abbreviations: PSM Propensity Score Matching, OR Odds Ratio, CI Confidence Interval, CDC Clavien-Dindo Classification, IQR Interquartile Range, PCD Percutaneous Catheter Drainage, ICU Intensive Care Unit
Follow-up
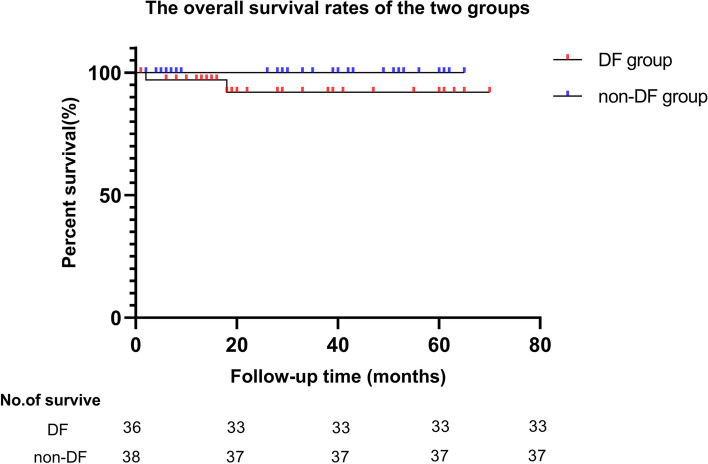
After PSM, no significant difference was observed in the follow-up time between the two groups (P > 0.05). Six cases of in-hospital mortality were excluded from follow-up analysis (four in the DF group and two in the non-DF group), leaving a total of 74 cases for follow-up. During this period, 2 cases were lost to follow-up (one patient in each group) and two died (both in the DF group). No significant difference was observed in the overall survival rate between the two groups (P > 0.05; Fig. 2). The incidence of events during follow-up, such as endocrine and exocrine insufficiency and pseudocysts of the pancreas, was not significantly different between the two groups (P > 0.05; Table 3).
Fig. 2.
The survival rates of the two groups. Note: The comparison of overall survival rates between duodenal and non-duodenal group. There was no significant statistical difference in survival rates between the two groups (P = 0.115)
Table 3.
Comparison of follow-up results between the two groups after PSM
| Outcome indicators | Duodenal Fistula Group(n = 36) | non-Duodenal Fistula Group (n = 38) | OR (95%CI) /Z | P-value |
|---|---|---|---|---|
|
Follow-up time (months) [Median (IQR)] |
14.5 (6.0–34.5) | 21.0 (5.0–42.0) | -0.721 | 0.471 |
| Follow-up events [n (%)] | ||||
| Pancreatic pseudocyst | 2 (5.5) | 6 (15.8) |
0.314 (0.059, 1.669) |
0.263 |
| Recurrent pancreatitis | 2 (5.5) | 4 (10.5) |
0.500 (0.086, 2.914) |
0.675 |
| Chronic pancreatitis | 1 (2.8) | 1 (2.6) |
1.057 (0.064, 17.560) |
1.00 |
| Pancreatic exocrine dysfunction | 8 (22.2) | 7 (18.4) |
1.265 (0.406, 3.940) |
0.684 |
| Pancreatic endocrine dysfunction | 5 (13.9) | 6 (15.8) |
0.860 (0.238, 3.111) |
0.818 |
Abbreviations PSM Propensity Score Matching, OR Odds Ratio, CI Confidence Interval, IQR Interquartile Range.\
Surgical interventions
Of the 197 patients, twenty (10.1%) underwent emergency open surgical debridement owing to intra-abdominal bleeding. 36 (18.3%) patients showed clinical improvement solely with continuous PCD without the need for surgical debridement; 141 (71.6%) patients were treated with PCD + LRPN, with a low conversion rate to open surgery (6.38%) (Table 4).
Table 4.
The treatment and etiology of enrolled patients
| The treatment methods (DF + non-DF) | n (%) |
|---|---|
| PCD alone | 36 (18.3) |
| PCD + LRPN | 141 (71.6) |
| Only once LRPN | 111 (78.7) |
| Conversion ratio of LRPN | 9 (6.38) |
| Open debridement | 20 (10.1) |
| Etiology of DF | |
| Iatrogenic | |
| Perforation due to nutrient tube | 7 (16.7) |
| Debridement or drainage tube compression (before the LRPN) | 5 (11.9) |
| PCD tube perforation | 1 (2.4) |
| Non-iatrogenic | |
| Complications of pancreatitis | 29 (69.0) |
| The diagnose of DF | |
| Endoscopy | 12 (28.6) |
| Fistulography | 10 (23.8) |
| Confirmed by surgery | 20 (47.6) |
Abbreviations DF Duodenal Fistula, PCD Percutaneous Catheter Drainage, LRPN Laparoscopic Retroperitoneal Pancreatic Necrosectomy
Regarding DF, diagnostic methods for DF included endoscopic examination, sinus tract radiography, and intraoperative discovery. Approximately half (47.6%) of DF cases were diagnosed during surgical debridement: 31% originated from iatrogenic injuries, such as duodenal perforation caused by feeding tubes or PCD tubes, or owing to prolonged compression by double-sleeve tubes on the duodenal wall (Table 4).
A comparative analysis of treatment modalities between the two groups revealed that a higher proportion of patients in the non-DF group showed clinical improvement solely with PCD (22.6% vs. 2.4%, P < 0.05), whereas a higher proportion of patients in the DF group underwent PCD + LRPN (88.1% vs. 67.1%, P < 0.05). No significant differences were observed between the two groups in the success rate of LRPN with single debridement or the rate of open surgical debridement (P > 0.05). However, the conversion rate to open surgery was higher in the DF group than in the non-DF group (13.5% vs. 3.8%), although not significant (P > 0.05; Table 5).
Table 5.
Comparison of treatment between DF and non-DF group
| The treatment methods | DF Group (n = 42) | non-DF Group (n = 155) | OR (95%CI) | P-value |
|---|---|---|---|---|
| PCD alone | 1 (2.4) | 35 (22.6) |
0.084 (0.011, 0.630) |
0.003* |
| PCD + LRPN | 37 (88.1) | 104 (67.1) |
3.629 (1.345, 9.787) |
0.007* |
| Only once LRPN | 29 (78.4) | 82 (78.8) |
0.973 (0.390, 2.424) |
0.952 |
| Conversion to open debridement | 5 (13.5) | 4 (3.8) |
3.906 (0.989, 15.430) |
0.053 |
| Open debridement | 4 (9.5) | 16 (10.3) |
0.914 (0.268, 2.896) |
1.00 |
*P < 0.05
Abbreviations DF Duodenal Fistula, OR Odds Ratio, CI Confidence Interval, PCD Percutaneous Catheter Drainage, LRPN Laparoscopic Retroperitoneal Pancreatic Necrosectomy
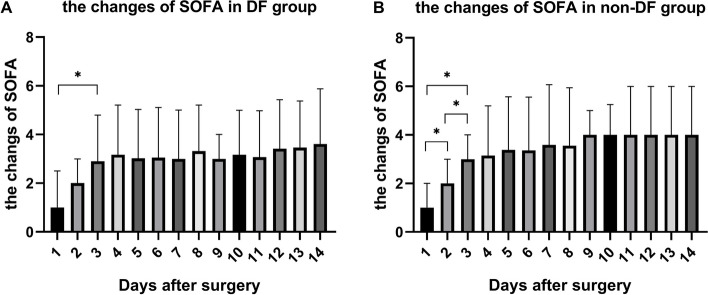
For patients undergoing LRPN debridement, SOFA scores were recorded daily for one day preoperatively and 15 days postoperatively. Within 72 h postoperatively, a significant decrease was observed in SOFA scores. After 72 h, the rate of decrease in SOFA scores slowed, gradually approaching stability (Fig. 3).
Fig. 3.
The changes of SOFA of the two groups. * p < 0.05
Discussion
DF is a severe complication in patients with IPN; its incidence ranks second only to that of colonic fistulas [16]. The relatively common occurrence of DF in patients with IPN is strongly associated with the anatomical location and underlying factors. These factors include corrosive effects, [17] compression of necrotic material around the pancreas, [18] and stimulation of various inflammatory factors induced by pancreatic necrosis that can lead to thrombosis in adjacent mesenteric vessels, thereby affecting the duodenal blood supply [19]. In addition, DF caused by iatrogenic injury should not be overlooked. This study found that approximately 30% of DF cases were related to iatrogenic injury, such as improper placement of double-sleeve tubes leading to prolonged pressure on the duodenal wall, improper placement of the PCD under ultrasound or CT guidance resulting in duodenal injury, and improper placement of nasojejunal feeding tubes causing damage to the duodenal mucosa. Excessive traction and separation during surgical debridement can cause DF. Bradley et al. reported that multiple surgical debridements increased the likelihood of gastrointestinal fistulas [20]. A systematic review reported that the incidence of PCD-related complications is approximately 2.5% in patients with PCD-treated pancreatitis [21]. Some patients in this study developed DFs after debridement at local hospital, indicating that both the debridement process and the placement of double-sleeve tubes after debridement may be risk factors for DF.
Common diagnostic methods for DF include endoscopic examination, sinus tract or upper gastrointestinal radiography, and intraoperative confirmation of DF during debridement. Some studies have reported that DF is often confirmed using sinus tract radiography [22]. However, at our center, DF is mostly diagnosed intraoperatively during debridement. Some patients are suspected of having an intestinal fistula before surgery, but endoscopic examination carries certain risks. When PCD drainage proves ineffective and the infection worsens, LRPN debridement and drainage are performed, often leading to the diagnosis of a duodenal fistula during surgery.
Regarding the treatment of IPN with DF, successful nonsurgical treatments are being used in many cases [16]. However, in such cases, in addition to the infectious fluid around the fistula, there is often a solid or semi-solid necrotic material. Pure drainage alone is ineffective and healing of the fistula often requires a long course of treatment, which is not conducive for rapid recovery. It is well known that gastrointestinal fistulas significantly increase the complexity of patient’s conditions and worsen their prognosis. However, after matching analysis, we found that the proportion of patients undergoing surgical debridement and the number of PCD and surgical procedures were higher in the DF group than in the non-DF group. This was based on clinical treatment needs of patients and their inevitable choices after discussion with the multidisciplinary team. At our center, more patients with DF underwent PCD with minimally invasive surgical debridement, whereas more patients without DF underwent PCD alone. However, no significant difference was observed in mortality or the incidence of severe complications between the two groups. This indicates that, for patients in the DF group, pure drainage may not be effective because of the more complex pancreatic infection. Instead, a step-up treatment strategy that combines PCD with minimally invasive debridement can effectively improve clinical symptoms and patient prognosis.
Surgical debridement of IPN has undergone significant developmental changes over the past few decades. Traditional open debridement to effectively remove necrotic material around the duodenum often requires dissection and freedom of the duodenum, which can exacerbate intra-abdominal infections. Moreover, open debridement involves a large incision, which often leads to difficulties in closing the abdomen owing to tissue edema and increased intra-abdominal pressure, resulting in a higher incidence of complications, such as wound infection and an increased risk of multi-organ failure, thereby affecting patient prognosis. A single-center study reported that regardless of the presence of concurrent intestinal fistulae, the mortality rate with open debridement exceeded 20% [16]. With the development of minimally invasive surgery and widespread application of the concept of damage control, various minimally invasive debridement techniques have greatly improved clinical outcomes in IPN. The evidence-based guidelines for acute pancreatitis treatment issued by the International Association of Pancreatology and the American Pancreatic Association in 2013 stated that for symptomatic IPN, minimally invasive debridement is superior to open debridement [23]. Therefore, our center prefers minimally invasive debridement for patients with IPN, regardless of the presence of DF.
Common minimally invasive debridement techniques include video-assisted retroperitoneal debridement [24], endoscopic transgastric necrosectomy [25], laparoscopic transgastric necrosectomy [26, 27], and rigid nephroscope assisted minimal access retroperitoneal pancreatic necrosectomy (MARPN) [28]. As a new minimally invasive treatment, LRPN, which is used in our center, is a modification of MARPN that combines the advantages of traditional open debridement and laparoscopy. Compared with traditional transabdominal debridement, retroperitoneal debridement is more direct, eliminating the need to dissect pancreatic ligaments and mobilize peripancreatic organs, thereby reducing the risk of intra-abdominal contamination. Additionally, the incision size in our center was approximately 2 cm, which is smaller than reported incisions for retroperitoneal debridement (5–7 cm) [24]. Although rigid nephroscope assisted or endoscopic minimally invasive debridement techniques result in smaller incisions, the efficiency of debridement is often low because of the characteristics of instruments that require repeated debridement. In contrast, LRPN with laparoscopic assistance achieves higher efficiency by using forceps for small incisions for simultaneous debridement and laparoscopic instrument debridement. Liu et al. achieved only 54% single debridement with a minimally invasive retroperitoneal approach via nephroscopy [29], whereas in our study, the single debridement rate exceeded 78%. LRPN far surpasses traditional MARPN in terms of debridement efficiency. Therefore, LRPN not only achieves thorough debridement and effective drainage but also reduces patient trauma and ensures debridement efficiency, aligning more with the principles of injury control and accelerated surgical recovery.
In this study, among patients treated with PCD + LRPN, regardless of whether they had concomitant DF, approximately 80% showed significant improvement in clinical symptoms after only one debridement, with lower rates of conversion to open surgery and overall mortality compared with data reported by other pancreatic centers domestically and internationally [30, 31]. Our overall mortality rate was similar to that reported by the Liverpool team [29]. After PSM matching, comparative analysis of clinical outcomes between the two groups found no significant differences in in-hospital mortality, long-term survival rate during follow-up, occurrence of follow-up events, rate of abdominal bleeding, and rate of postoperative severe complications (CDC ≥ 3). The SOFA scores within 72 h postoperatively were significantly lower in both groups, with similar decreasing trends. Therefore, we believe that the low mortality rate and low incidence of complications in IPN patients with DF are closely related to the safe and efficient debridement of LRPN as well as the rapid improvement in organ dysfunction after LRPN.
This research primarily examines the effectiveness and safety of LRPN in treating IPN, focusing on postoperative complications and clinical outcomes, with satisfactory results. However, it does not address changes in inflammatory markers or enzymes that reflect infection severity. Research has shown that indicators such as interleukin-6 (IL-6) and butyrylcholinesterase (BChE) can assess infection severity and evaluate the effectiveness of anti-infection treatments [32, 33]. Therefore, in the future, the research team plans to design more scientifically rigorous studies incorporating inflammatory markers to further validate the effectiveness and safety of LRPN in treating IPN.
Our study had some limitations. As a retrospective analysis, this study lacked a control group; therefore, data reliability was insufficient. Therefore, in future research, we plan to design randomized controlled trials related to LRPN to fully confirm the advantages of LRPN in treating IPN. The data used in this study were obtained from historical records. However, some records may be incomplete or inaccurate, leading to bias. As this study involved reviewing data from previous years, changes in clinical diagnosis and treatment processes may have affected the reliability of the results. After PSM, the sample size of both groups was relatively small (n = 40), which may have led to selection bias and affected the reliability of the conclusions.
Conclusions
DF may complicate the condition of patients with IPN, potentially making minimally invasive treatments more challenging. However, the results of the retrospective cohort study indicate that LRPN-centered step-up approach is both safe and effective for patients with IPN and DF.
Supplementary Information
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
Abbreviations
- CDC
Clavien–Dindo Classification
- DF
Duodenal fistula
- IPN
Infected pancreatic necrosis
- LRPN
Laparoscopic retroperitoneal pancreatic necrosectomy
- PCD
Percutaneous catheter drainage
- PSM
Propensity score-matching
- SOFA
Sequential Organ Failure Assessment
Authors’ contributions
Study conception and design: BS and HY. Collection and assembly of data: RRW and YMH. Statistical analysis and interpretation of data: RRW and YMH. Wrote the manuscript: RRW. Reviewed and revised the article: YFT, BS and XY. All authors reviewed the manuscript and approved the final version to be published.
Funding
This study was supported by Science and Technology Program of Zhejiang Province (2024C03201), Co-construction science and technology program of Zhejiang Traditional Chinese Medicine Administration (GZY-ZJ-KJ-24032), The Zhejiang Provincial Medical and Health Science and Technology Plan (2020RC076) and National Natural Science Foundation of China (No. 82270670).
Data availability
The datasets that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the Sir Run Run Shaw Hospital Ethics Committee (No. 2024–0214). The clinical data of all enrolled patients were obtained from the clinical information system and analyzed anonymously, and the informed consents were waived approved by the ethics committees of the Sir Run Run Shaw Hospital.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Bo Shen, Email: simpleshen@zju.edu.cn.
Hong Yu, Email: blueyu000@zju.edu.cn.
References
- 1.Iannuzzi J, King J, Leong J, Quan J, Windsor J, Tanyingoh D, et al. Global incidence of acute pancreatitis is increasing over time: a systematic review and meta-analysis. Gastroenterology. 2022;162(1):122–34. [DOI] [PubMed] [Google Scholar]
- 2.Mederos M, Reber H, Girgis M. Acute pancreatitis: a review. JAMA. 2021;325(4):382–90. [DOI] [PubMed] [Google Scholar]
- 3.Forsmark C, Vege S, Wilcox C. Acute Pancreatitis. N Engl J Med. 2016;375(20):1972–81. [DOI] [PubMed] [Google Scholar]
- 4.Boxhoorn L, Voermans R, Bouwense S, Bruno M, Verdonk R, Boermeester M, et al. Acute pancreatitis. Lancet (London, England). 2020;396(10252):726–34. [DOI] [PubMed] [Google Scholar]
- 5.Timmerhuis H, van Dijk S, Hollemans R, Umans D, Sperna Weiland C, Besselink M, et al. Perforation and fistula of the gastrointestinal tract in patients with necrotizing pancreatitis: a nationwide prospective cohort. Ann Surg. 2023;278(2):e284–92. [DOI] [PubMed] [Google Scholar]
- 6.Jiang W, Tong Z, Yang D, Ke L, Shen X, Zhou J, et al. Gastrointestinal fistulas in acute pancreatitis with infected pancreatic or peripancreatic necrosis: A 4-year single-center experience. Medicine (Baltimore). 2016;95(14):e3318. [DOI] [PMC free article] [PubMed]
- 7.Singh A, Aggarwal M, Garg R, Walsh M, Stevens T, Chahal P. Spontaneous internal pancreatic fistulae complicating acute pancreatitis. Am J Gastroenterol. 2021;116(7):1381–6. [DOI] [PubMed] [Google Scholar]
- 8.Lu J, Zheng Z, Ding Y, Qu Y, Mei W, Fang Z, et al. Characteristics and Incidence of Colon Complication in Necrotizing pancreatitis: A Propensity Score-Matched Study. J Inflamm Res. 2023;16:127–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Santvoort H, Besselink M, Bakker O, Hofker H, Boermeester M, Dejong C, et al. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med. 2010;362(16):1491–502. [DOI] [PubMed] [Google Scholar]
- 10.van Brunschot S, Hollemans R, Bakker O, Besselink M, Baron T, Beger H, et al. Minimally invasive and endoscopic versus open necrosectomy for necrotising pancreatitis: a pooled analysis of individual data for 1980 patients. Gut. 2018;67(4):697–706. [DOI] [PubMed]
- 11.Erik vE, Douglas G A, Matthias E, Stuart J P, Peter C G, Jan P VJIJS. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014;12. [DOI] [PubMed]
- 12.World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–4. [DOI] [PubMed]
- 13.Banks P, Bollen T, Dervenis C, Gooszen H, Johnson C, Sarr M, et al. Classification of acute pancreatitis–2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62(1):102–11. [DOI] [PubMed] [Google Scholar]
- 14.Leppäniemi A, Tolonen M, Tarasconi A, Segovia-Lohse H, Gamberini E, Kirkpatrick A, et al. WSES guidelines for the management of severe acute pancreatitis. World J Emerg Surg. 2019;2019(14):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Santvoort H, Besselink M, Bakker O, Hofker H, Boermeester M, Dejong C, et al. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med. 2010;362(16):1491–502. [DOI] [PubMed] [Google Scholar]
- 16.Wei J, Zhihui T, Dongliang Y, Lu K, Xiao S, Jing Z, et al. Gastrointestinal Fistulas in Acute Pancreatitis With Infected Pancreatic or Peripancreatic Necrosis: A 4-Year Single-Center Experience. Medicine (Baltimore). 2016;95. [DOI] [PMC free article] [PubMed]
- 17.Hua Z, Su Y, Huang X, Zhang K, Yin Z, Wang X, et al. Analysis of risk factors related to gastrointestinal fistula in patients with severe acute pancreatitis: a retrospective study of 344 cases in a single Chinese center. BMC Gastroenterol. 2017;17(1):29. [DOI] [PMC free article] [PubMed]
- 18.Maatman T, Nicolas M, Roch A, Lewellen K, Al-Azzawi H, Ceppa E, et al. Colon involvement in necrotizing pancreatitis: incidence risk factors, and outcomes. Ann Surg. 2022;275(3):568–75. [DOI] [PubMed] [Google Scholar]
- 19.Rana SS, Sharma R, Dhalaria L, Kang M, Gupta R. A case series of late gastrointestinal fistulization in 16 patients with walled-off necrosis. Dig Dis Sci. 2021;67(2):661–6. [DOI] [PubMed] [Google Scholar]
- 20.Edward L 3rd B, Nadine D DJAS. Management of severe acute pancreatitis: a surgical odyssey. Ann Surg. 2009;251. [DOI] [PubMed]
- 21.M C vB, H C vS, T L B, O J B, M G B, H G GJBJS. Systematic review of percutaneous catheter drainage as primary treatment for necrotizing pancreatitis. Br J Surg. 2010;98. [DOI] [PubMed]
- 22.Zhang J, Yang J, Li G. Management of duodenal fistulas in infected pancreatic necrosis patients: A propensity score matching. Chin J Pract Surg. 2021;41(3):315–9. [Google Scholar]
- 23.Guidelines. WGIAAP. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13:e1–15. [DOI] [PubMed]
- 24.van Santvoort H, Besselink M, Horvath K, Sinanan M, Bollen T, van Ramshorst B, et al. Videoscopic assisted retroperitoneal debridement in infected necrotizing pancreatitis. HPB (Oxford). 2007;9(2):156–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seifert H, Biermer M, Schmitt W, Jürgensen C, Will U, Gerlach R, et al. Transluminal endoscopic necrosectomy after acute pancreatitis: a multicentre study with long-term follow-up (the GEPARD Study). Gut. 2009;58(9):1260–6. [DOI] [PubMed]
- 26.Worhunsky D, Qadan M, Dua M, Park W, Poultsides G, Norton J, et al. Laparoscopic transgastric necrosectomy for the management of pancreatic necrosis. J Am Coll Surg. 2014;219(4):735–43. [DOI] [PubMed] [Google Scholar]
- 27.Driedger M, Zyromski N, Visser B, Jester A, Sutherland F, Nakeeb A, et al. Surgical transgastric necrosectomy for necrotizing pancreatitis: a single-stage procedure for walled-off pancreatic necrosis. Ann Surg. 2020;271(1):163–8. [DOI] [PubMed] [Google Scholar]
- 28.Shen D, Ning C, Huang G, Liu Z. Outcomes of infected pancreatic necrosis complicated with duodenal fistula in the era of minimally invasive techniques. Scand J Gastroenterol. 2019;54(6):766–72. [DOI] [PubMed] [Google Scholar]
- 29.Gomatos I, Halloran C, Ghaneh P, Raraty M, Polydoros F, Evans J, et al. Outcomes from minimal access retroperitoneal and open pancreatic necrosectomy in 394 patients with necrotizing pancreatitis. Ann Surg. 2016;263(5):992–1001. [DOI] [PubMed] [Google Scholar]
- 30.Liu ZW, Yang SZ, Wang PF, Feng J, He L, Du JD, et al. Minimal-access retroperitoneal pancreatic necrosectomy for infected necrotizing pancreatitis: a multicentre study of a step-up approach. Br J Surg. 2020;107. [DOI] [PubMed]
- 31.Hjalmar C vS, Olaf J B, Thomas L B, Marc G B, Usama AA, A Marjolein S, et al. A conservative and minimally invasive approach to necrotizing pancreatitis improves outcome. Gastroenterology. 2011;141. [DOI] [PubMed]
- 32.van den Berg F, de Bruijn A, van Santvoort H, Issa Y, Boermeester MJPojotIAoP. Early laboratory biomarkers for severity in acute pancreatitis; A systematic review and meta-analysis. Pancreatology. 2020;20(7):1302–11. [DOI] [PubMed]
- 33.Verras G. Mulita FJFis. Butyrylcholinesterase levels correlate with surgical site infection risk and severity after colorectal surgery: a prospective single-center study. Front Surg. 2024;11:1379410. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request.