Abstract
Heart failure with preserved ejection fraction (HFpEF), a complex disease that is increasingly prevalent due to population aging, pose significant challenges in its treatment. The present study utilized the HFpEF rat model and H9C2 cells as research subjects to thoroughly investigate the potential mechanisms of alarin in protecting cardiac function in HFpEF. The study shows that under HFpEF conditions, oxidative stress significantly increases, leading to myocardial structural damage and dysfunction of calcium ion channels, which ultimately impairs diastolic function. Alarin, through its interaction with NADPH oxidase 1 (NOX1), effectively alleviates oxidative stress and modulates the activities of type 2 ryanodine receptor (RyR2) and sarcoplasmic/endoplasmic reticulum calcium ATPase 2 (SERCA2), thereby facilitating the restoration of Ca2+ homeostasis and significantly improving cardiac function in the HFpEF model. This research not only uncovers the cardioprotective effects of alarin and its underlying molecular mechanisms but also provides new insights and potential therapeutic targets for HFpEF treatment strategies, suggesting a promising future for alarin and related therapies in the management of this debilitating condition.
Key words: alarin, HFpEF, oxidative stress, NOX1, calcium ion channels
Introduction
Heart failure with preserved ejection fraction (HFpEF) is a common heart disease characterized by normal contractile function but impaired diastolic function, leading to compromised systemic blood flow.1 Despite maintaining an ejection fraction (EF) exceeding 50%, patients with HFpEF commonly experience dyspnea, fatigue, edema, and reduced exercise tolerance, all of which severely diminish their quality of life.2-4 As the population ages and lifestyles transform, the occurrence of HFpEF is escalating.5 Consequently, research on HFpEF holds paramount significance in preventing and managing this increasingly prevalent condition.
Recent advancements in heart failure (HF) research have underscored the central role of oxidative stress in disease initiation and progression.6,7 Oxidative stress triggers the accumulation of reactive oxygen species (ROS) within cardiomyocytes, igniting a cascade of biochemical reactions, including protein oxidation and lipid peroxidation.8,9 These reactions disrupt cellular structures and functions, contributing to myocardial cell damage and dysfunction.10 Notably, NADPH oxidase (NOX), a family of enzymes that catalyze the production of ROS, plays a pivotal role in modulating oxidative stress level.11 The NOX family consists of seven members, including NOX1, NOX2, NOX3, NOX5, and DUOX oxidases, which regulate oxidative stress through intracellular signal transduction.12,13
HFpEF is primarily defined by left ventricular diastolic dysfunction, arising from an imbalance Ca2+ handling within the myocardial sarcoplasmic reticulum (SR).14 This imbalance leads to intracellular Ca2+ overload, which is exacerbated by ROS-mediated enhancement of RyR2 (type 2 ryanodine receptor, a calcium release channel located on the SR membrane of cardiomyocytes) activity and suppression of SERCA2 (sarcoplasmic/endoplasmic reticulum calcium ATPase 2, a calcium pump responsible for pumping cytosolic Ca2+ back into the SR) function.15-18 The resultant disturbance in Ca2+ homeostasis facilitates troponin displacement, causing actin and myosin to remain bound, thereby perpetuating myocardial contraction and diastolic dysfunction.19
Despite recent progress in HFpEF treatment, including the use of sodium-glucose co-transporter 2 (SGLT2) inhibitors, mineralocorticoid receptor antagonists (MRAs), angiotensin receptor neprilysin inhibitors (ARNIs), and beta-blockers (BBs), there are still significant limitations to these therapies.20-22 The overall efficacy of these drugs is often debated, and they may not be universally effective or may come with undesirable side effects. Thus, there is an urgent need for novel therapeutic approaches to address the unmet medical needs of HFpEF patients.
Alarin, a neuropeptide belonging to the galanin peptide family, has garnered attention among researchers due to its extensive biological activities.23 Composed of 25 amino acids, alarin exerts its effects through GalR1, GalR2, and GalR3 receptors, which are members of the G protein-coupled receptor family and play a vital role in various pathological processes, including cardiovascular diseases.24-26 Our previous studies have demonstrated that alarin can effectively inhibit the angiotensin II (Ang II)-induced upregulation of NOX1 expression in cardiac fibroblasts,27 and it has shown promise in improving cardiac function and mitigating myocardial fibrosis in rat models of acute myocardial infarction (AMI). Furthermore, proteomic analyses have revealed a significantly reduced alarin levels in the serum of HFpEF rats, suggesting a potential role for alarin in this disease. However, the precise mechanism and therapeutic potential of alarin in HFpEF remain to be fully elucidated and warrant further investigation.
In this study, to investigate the role of alarin in HFpEF, an in vitro model of H9C2 cells treated with isoproterenol and an in vivo model of Dahl rats fed a high-salt diet (HSD) were established. By investigating the improvement effects of alarin on cardiac function in HFpEF and the possible mechanisms by measuring cardiac function, oxidative stress level, the expression levels of alarin and NOX1, the interaction between alarin and NOX1 as well as the expression of RyR2 and SERCA2, the aim of this study is to provide a theoretical basis for exploring novel treatment.
Materials and Methods
Animals and experimental protocol
The male Dahl rats (220-240 g, 6-week-old) were purchased from the Institute of Comparative Medicine (Yangzhou University, Yangzhou, China), and housed in a specific pathogen-free environment. The rats were housed in a temperature-regulated environment, maintained on a regular 12 h light-dark cycle, and provided with unrestricted access to standard food and drinking water. The animal experiments were approved by the Experimental Animal Care and Use Committee of Xuzhou Medical University (Approval no. SCXK (Su)2022-0009). After one week of adaptive feeding, the model of HFpEF was constructed using a high-salt diet (diet with 8% NaCl, HSD) for 7 weeks, and using echocardiographic observation to confirm the successful establishment of the HFpEF model.
The rats were randomly divided into six groups as follows: i) control group: normal rats received daily intraperitoneal injections of saline; ii) HSD group: the HFpEF model rats also received daily intraperitoneal injections of saline; iii) alarin group: the model rats received intraperitoneal injections of alarin (1 nM/kg/d);27 iv) EXNC group: the model rats were intraperitoneally administered with overexpression control vector lentivirus once weekly; v) EXNOX1 group: the model rats were intraperitoneally administered with NOX1 overexpression vector lentivirus once weekly; vi) alarin + EX-NOX1 group: the model rats received intraperitoneal administrations of alarin (1 nM/kg/d) and NOX1 overexpression vector lentivirus once weekly. After four weeks of intervention, echocardiographic evaluation was conducted to detect changes in cardiac function among rats in each group. At the end of the experiment, the rats were euthanised by cervical dislocation, and the myocardial tissue was isolated for the following experiments.
Histopathological analysis
The cardiac muscle tissue was fixed in 4% paraformaldehyde (AR1069; Boster Bio, Pleasanton, CA, USA) and subsequently sliced into 4 μm-thick sections measuring, and then stained with hematoxylin and eosin (H&E) (C0105M; Beyotime Biotech, Inc., Haimen, China) and Masson’s trichrome (G1340; Solarbio, Beijing, China) stain for histopathological analysis.
Cells and culture
The rat cardiomyocyte cell line, H9C2 cells, obtained from Fenghui Biotech (Hunan, China) were cultured in DMEM medium (PM150210; Pricella Biotech, Houston, TX, USA) containing 10% FBS (164210; Pricella Biotech) and 1% penicillin-streptomycin solution (PB180120; Pricella Biotech) at 37°C, in 5% CO2. To establish a H9C2 cell injury model, cells were treated with isoproterenol (ISO) at a concentration of 0.1 mg/mL for a duration of 24 h. Then the cells were divided into six groups: control group, in which the normal H9C2 cells were treated with PBS; model group, in which the model cells were treated with PBS; alarin group, the model cells were treated with alarin (10 nM); EX-NC group, the model cell which transfected the overexpression control vector were treated with PBS. EX-NOX1 group, the model cell which transfected the NOX1 overexpression vector were treated with PBS. Alarin + EX-NOX1 group, the model cell which transfected the NOX1 overexpression vector were treated with alarin (10 nM). All the groups of cells were incubator under 37°C and 5% CO2 conditions for 48 h, followed by the subsequent experiments.
ELISA testing for level of oxidative stress
Commercial ELISA kit for ROS (YFXER00746; Yfxbio Biotech, Nanjing, China) and 8-hydroxy-2’-deoxyguanosine (8OHdG) (ab285254; Abcam, Cambridge, UK) was used to determine the concentrations of ROS and 8-OHdG in the cardiac muscle tissue and H9C2 cells, in accordance with the manufacturer’s protocols.
Viability analysis
Cell viability was detected using a Cell Viability Assay Kit (CCK-8, C0037; Beyotime Biotech, Inc.). In the presence of electron-coupled reagents, intracellular dehydrogenases can oxidize it to generate a water-soluble orange formazan. The quantity of formazan produced is directly proportional to the number of viable cells present. According to the manufacturer’s protocols, each group cells were seeded in 96-wellplates and incubated for 48 h. Then, the cells were exposed to the drug for 4 h, followed by the addition of 10 μL of CCK-8 solution per well for a further 2 h incubation. Finally, the absorbance was measured at 450 nm by microplate reader (Multiskan 51119000; Thermo Fisher Scientific, Waltham, MA, USA).
Intracellular ROS analysis
We used DCFH-DA (S0033S; Beyotime Biotech, Inc.) to measure ROS accumulation in H9C2 cells. According to the manufacturer’s protocols, cells from each group were incubated for 24 h in 96-well plates, followed by a 20 min incubation with a final concentration of 10 μM DCFH-DA. Subsequently, the fluorescence intensity of DCF was assayed using a fluorescence microscope (CKX31; Olympus, Tokyo, Japan) with an excitation wavelength of 488 nm and an emission wavelength of 525 nm, and the corresponding images were captured.
ELISA testing for alarin
ELISA assay for extracellular the level of alarin in each group of cells were determined using alarin (Rat) ELISA kit (EK-026-33; Phoenix Pharmaceuticals, Burlingame, CA, USA) according to the manufacturer’s instructions.
Western blot analysis
The cells were lysed on ice in a cell lysis buffer, and protein extracts were obtained through centrifugation at 12,000 g for 30 min at 4°C. The concentrations of these proteins were accurately determined using the bicinchoninic acid (BCA) Protein Assay kit (P0010; Beyotime Biotech, Inc.). Subsequently, equivalent quantities of protein extracts were separated by 10% SDS-PAGE gel electrophoresis and then transferred onto PVDF membranes (ISEQ00010; Millipore, Burlington, MA, USA). Prior to antibody binding, the membranes were blocked with 5% skimmed milk for a duration of 2 h. This was followed by an overnight incubation with primary antibodies (alarin: PA5-62877, Thermo Fisher Scientific, dilution: 1:500; NOX1: ab131088, Abcam, dilution: 1:500; RYR2: ab302716, Abcam, dilution: 1:1000; SERCA2, ab150435, Abcam, dilution: 1:1000; β-actin: ab8227, Abcam, dilution: 1:2000) at 4°C, which was then succeeded by a 2 h incubation with the corresponding secondary antibody (IgG H&L (HRP), ab6721, Abcam, dilution: 1:1000) at room temperature. Finally, the bands were visualized via ECL (180-5001; Tanon Science & Technology Co., Ltd., Shanghai, China) and detected by Chemiluminescence Detection System (5200; Tanon Science & Technology Co., Ltd.). The gray values of the protein bands were analyzed using ImageJ software and β-actin was used as a control.
Immunohistochemistry
The myocardial tissues were fixed using paraformaldehyde and subsequently sliced into 4 μm-thick sections. These sections were then freed from paraffin and dehydrated through a series of xylene and graded alcohol treatments. After heating the 1×EDTA antigen retrieval solution in a microwave until boiling, the slides were adjusted to low heat for 15 min and then allowed to cool naturally; they were then washed with PBS three times (5 min each). Overnight incubation at 4°C was conducted with rabbit anti-alarin (PA5-62877, Thermo Fischer Scentific; dilution: 1:500) and antiNOX1 (ab131088, Abcam; dilution: 1:500) antibodies (PBS buffer instead of the primary antibody was used as a negative control). Subsequently, the sections were incubated with an HRP-conjugated goat anti-rabbit secondary antibody (IgG H&L (HRP), ab6721, Abcam; dilution: 1:1000) for 1 h at room temperature. The sections were then stained with DAB staining solution revealing the presence and localization of the antigens, and counterstained with hematoxylin. Finally, the staining outcomes were carefully inspected using a light microscope, and the expression of the target antigen was qualitatively analyzed by observing the staining intensity and distribution pattern.
Co-immunoprecipitation assays
H9C2 cells were collected and lysed with RIPA lysis buffer (R0010; Solarbio), followed by centrifugation at 12,000 g for 10 min at 4°C. To investigate the interaction between alarin and NOX1, the clarified supernatants were incubated overnight with proteinA/G-agarose (P2055; Beyotime Biotech, Inc.,) and an anti-alarin antibody (ab170923; Abcam), an anti-NOX1 antibody (ab131088; Abcam), or negative control IgG (ab172730; Abcam). Then, the immunoprecipitants underwent four thorough washes with RIPA lysis buffer, followed by boiling in 5×SDS loading buffer to prepare them for subsequent immunoblot analysis.
Statistical analyses
The data obtained in this study were analyzed utilizing the Graphpad Prism 8.02 software, and the results were presented as the mean ± SD. To assess differences between two groups, an unpaired Student’s t-test was employed, while for comparing differences across multiple groups, a one-way ANOVA with Tukey’s post-hoc test was conducted.
Results
Effects of alarin on cardiac function in rats with HFpEF
Firstly, echocardiographic assessments were performed to determine whether alarin treatment could enhance cardiac function in rats with HFpEF. Specifically, we measured the EF index, left atrial (LA) dimension, and E/e’ ratio, which are established markers of cardiac function. As shown in Figure 1A, compared with the Control group, the EF index in HSD group decreased significantly (p<0.0001), while LA and E/e’ indices displayed significant elevations (p<0.0001, p=0.0005 respectively). Conversely, both the alarin group and the alarin + EX-NOX1 group exhibited a marked increase in EF index (p=0.0006, p<0.0001) and a pronounced decrease in LA and E/e’ indices when compared to the HSD group (p=0.0023, p=0.0005). Meanwhile, alarin treatment was able to mitigate the structural abnormalities of myocardial cells (Figure 1B) and reduce the amount of collagen fibers in the myocardial tissue (Figure 1C). The overexpression of NOX1 exacerbated the structural disarray of myocardial cells and significantly increased the presence of collagen fibers, whereas alarin treatment exerted a certain degree of improvement. These results indicate that alarin can improve cardiac function in HFpEF rats and reverse the deleterious effects of the overexpression of NOX1.
Effects of alarin on oxidative stress in HFpEF
To elucidate the impact of alarin on oxidative stress in HFpEF, we quantitatively assessed the levels of ROS and 8-hydroxy-2’-deoxyguanosine (8-OHdG), markers of oxidative DNA damage, in myocardial tissue samples. As shown in Figure 2A, the content of ROS and 8-OHdG in the HSD group were significantly elevated (p<0.0001). However, treatment with alarin significantly reduced the levels of ROS and 8-OHdG (p<0.0001), indicating that alarin can decrease the oxidative stress level in HFpEF rat cardiomyocytes. The overexpression of NOX1 increases oxidative stress, but alarin can significantly reverse this effect. Similar results were obtained in H9C2 cells. The cell viability in the model group was significantly lower than that in the Control group, while alarin treatment improved cell viability (Figure 2B). Additionally, alarin treatment also significantly reduced the levels of 8-OHdG (Figure 2C) and ROS (Figure 2D) in the alarin group and alarin + EXNOX1 group cells.
Changes in expression level of alarin in HFpEF
To investigate the expression pattern of alarin in myocardial tissue of rats with HFpEF, immunohistochemical staining was performed. As shown in Figure 3A, compared to the control group, the HSD group exhibited lighter tan staining and reduced positive signals, suggestive of reduced alarin expression. Administration of alarin was able to elevate its expression level. Comparable observations were made in experiments involving H9C2 cells. As shown in Figure 3 B,C, the model group exhibited significantly lower levels of alarin expression in both cell supernatants and cells compared to the control group (p<0.0001). Notably, alarin treatment effectively increased alarin expression in both the cell supernatants and cells in the alarin group and alarin + EX-NOX1 group cells (p<0.0001).
Figure 1.
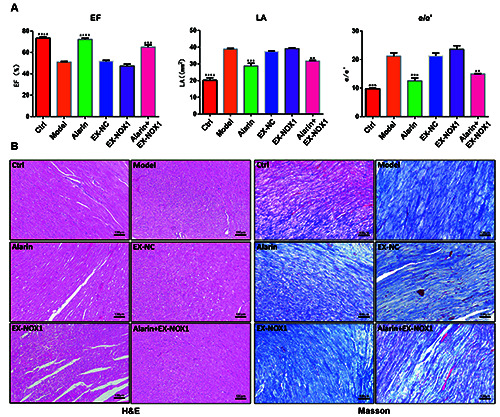
Alarin improved cardiac function in HFpEF rats. A) Echocardiographic examination of the EF, LA, and E/e' in HFpEF rats (n=5). B) Observation of H&E-stained cardiac tissue sections (n=3). C) Observation of Masson's trichrome-stained cardiac tissue sections (n=3). The results are expressed as mean ± SEM. EF, ejection fraction; LA, left atrium area; *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 indicate statistically significant difference versus the model group; scale bars: 100 μm.
Alarin can interacts with NOX1
The preceding results have confirmed the ability of alarin to reduce oxidative stress levels, and further discovered its potential to reverse the adverse effects of NOX1 overexpression in HFpEF. These indicate a possible interaction between alarin and NOX1. To confirm this interaction, we conducted co-immunoprecipitation assays, which demonstrated a direct interaction between alarin and NOX1 (Figure 4A). Moreover, compared to the control group (Figure 4B), the HSD group exhibited a deeper tan coloration, indicating an increase in positive signals and a higher expression level of NOX1. In contrast, the expression level of NOX1 in the alarin group was significantly reduced compared to the HSD group (p=0.0008), and the alarin + EX-NOX1 group also showed a marked decrease compared to the EX-NOX1 group (p=0.0007). Similar results were observed in H9C2 cells (Figure 4C). These results indicate that there is a significant increase in NOX1 levels in HFpEF, and alarin can effectively suppresses the expression of NOX1.
Figure 2.
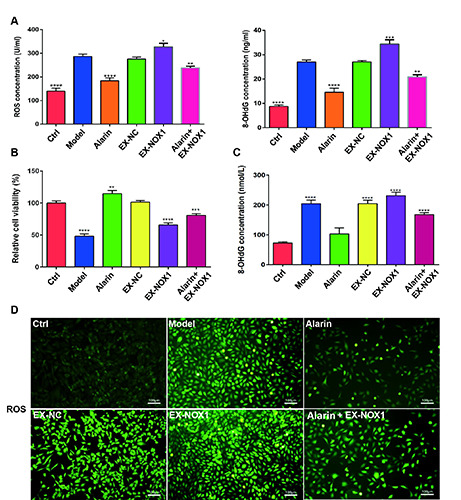
Alarin alleviated the oxidative stress in HFpEF. A) Content of ROS and 8-OHdG in myocardial tissue of HFpEF rats were assessed by ELISA. B) Viability of H9C2 cells in the different groups. C) The content of 8-OHdG in H9C2 cells was assessed by ELISA. D) Relative level of ROS detected by DCF probe in H9C2 cells; scale bars: 100 μm. The results are expressed as mean ± SEM; n=3 for each group. ROS, reactive oxygen species; 8-OHdG, 8-hydroxy-2'-deoxyguanosine; *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 indicate statistically significant difference versus the model group (A,B,D).
Effect of alarin on calcium ion channels in HFpEF
Extensive research has demonstrated that HFpEF is characterized by left ventricular diastolic dysfunction, primarily due to the enhancement of RyR2 activity and concurrent inhibition of SERCA2 activity by ROS, leading to an imbalance in intracellular Ca2+ levels. Our current investigation demonstrated that in myocardial tissue from HFpEF rats, there was a notable upregulation of RyR2 expression (p<0.0001) and concurrent downregulation of SERCA2 expression (p<0.0001), which is consistent with previous reports on the mechanisms underlying intracellular Ca2+ imbalance in HFpEF (Figure 5A). Notably, administration of alarin effectively reduced the expression of RyR2 (p=0.0326) and simultaneously upregulated the expression of SERCA2 (p=0.0046). Furthermore, overexpression of NOX1 led to a significant increase in RyR2 expression (p<0.0001) and a decrease in SERCA2 expression (p<0.0001); however, these alterations were reversed upon treatment with alarin. Additionally, similar results were obtained in experiments using H9C2 cells in vitro (Figure 5B).
Discussion
Alarin, a galanin peptide, exhibits a widespread distribution, suggesting its diverse array of physiological functions.28 It was found that alarin can improve HF and cardiac fibrosis via alleviating oxidative stress in HF rats.27 In this study, the protective effect of alarin against HFpEF and the possible underlying mechanism were investigated in rat and in cell models. We investigated the effects of the interaction between alarin and NOX1 on alleviating oxidative stress and subsequent changes in the activity of RyR2 and SERCA2 involved in regulating Ca2+ release and uptake in the sarcoplasmic reticulum of cardiomyocytes. These results indicated that alarin exhibits the capacity to improve cardiac function in HFpEF.
HFpEF is a complex syndrome that is continually increasing in incidence and proportion, driven by the trend of aging. Despite left ventricular EF remaining normal or near-normal range (typically greater than 50%), inefficient pumping of blood by the heart leads to poor circulation within the body. The main characteristics of HFpEF are structural changes in the heart, such as left ventricular hypertrophy or left atrial enlargement, as well as impaired left ventricular diastolic function.29 These changes result in the heart’s inability to adequately expand during diastole to accommodate sufficient blood, leading to a decrease in the heart’s filling volume. In the present study, we found that EF was reduced in HFpEF rats, and while LA and E/e’ indices increased significantly, which were reversed by alarin treatment. These results indicated that alarin can improve cardiac function in HFpEF. However, there is often associated abnormalities in skeletal muscle and adipose tissue in HFpEF, and whether alarin also plays a role in it remains to be further studied.30,31
Figure 3.
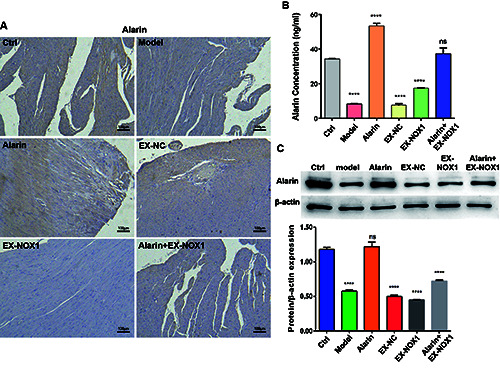
Administering alarin could improve the expression level of alarin in HFpEF rats and H9C2 cells. A) Immunohistochemistry was used to identify alarin expression in myocardial tissue; scale bar: 100 μm. B) Detection of alarin in the supernatant of H9C2 cells. C) Expression level of alarin in H9C2 cells. The results are expressed as mean ± SEM; n=3 for each group. ****p<0.0001; ns, not significant.
The pathogenesis of HFpEF is not fully understood at present. However, studies have shown that inflammation, oxidative stress, and myocardial fibrosis are involved in the occurrence and development of HFpEF.27,32,33 These factors usually interact together to cause changes in the structure and function of the heart, leading to heart failure. We found structural disturbances in the myocardial cells and a significant increase in collagen fibers in the cardiac tissues of HFpEF rats. This indicates that the cardiac tissues of HFpEF rats undergo changes in structure and function. Research has confirmed that long-term exposure of myocardial cells to oxidative stress leads to abnormal changes in cell structure and function.27 Oxidative stress, a hallmark of HFpEF, occurs due to an imbalance between the production of ROS and the antioxidant defense mechanisms. NOX is the main class of enzymes that induce the generation of ROS in the vascular system, transferring electrons from NADPH to oxygen molecules, resulting in the production of NADP+, superoxides, and other downstream ROS.13 The NOX family of proteins consists of seven members. The distribution of different members of the NOX family varies in tissues and cells. NOX1 is associated with cell growth and is mainly expressed in the colon, prostate, uterus, and vascular cells.11,12 It has been demonstrated that the oxidative stress mediated by NOX1 plays a crucial role in cardiovascular diseases. We found that NOX1 expression levels were elevated in HFpEF rats and H9C2 cells, leading to an increase in ROS and 8-OhdG levels, which were inhibited by alarin administration. Additionally, overexpression of NOX1 in HFpEF rats and H9C2 cells resulted in significantly increased oxidative stress levels, which were reversed by alarin treatment. Subsequently, we discovered that alarin can interact with NOX1. These results suggest that alarin reduces oxidative stress levels in HFpEF by binding with NOX1. Oxidative stress is an important pathological process in many cardiovascular diseases, including HFpEF. The binding of alarin and NOX1 improves the oxidative stress state through certain mechanisms, which may involve inhibiting the production of ROS and enhancing the antioxidant defense system, among others. Further research is needed to elucidate the specific mechanisms by which alarin and NOX1 binding improve oxidative stress.
Excitation-contraction coupling (ECC) in cardiac myocytes is an important mechanism for heart contraction.34 During each heartbeat, the depolarization of the cardiac myocyte membrane triggers the influx of Ca2+ through the CaV1.2 channels, activating the release of a large amount of Ca2+ from the SR via the RyR2 receptors, which induces the release of intracellular calcium and leads to myocardial contraction. During the diastolic phase, the intracellular Ca2+ concentration increases, which activates the SERCA2 receptors on the SR membrane to sequester Ca2+ back into the SR, or it can be pumped out of the cell by the NCX1 (Sodium-calcium exchanger 1) on the cell membrane, reducing the cytoplasmic Ca2+ concentration. HFpEF manifests as impairment of left ventricular diastolic function, primarily due to imbalances in the absorption and release of intracellular Ca2+ in the myocardial sarcoplasmic reticulum, resulting in Ca2+ overload in cardiac myocyte.16,18 The formation of complexes between excessive free Ca2+ and myofilament proteins displaces troponin, leading to the binding of actin and myosin, causing the myocardium to remain in a contracted state, thus impairing diastolic function.19 This study found that in HFpEF rats and H9C2 cells, the expression level of RyR2 increased, while the level of SERCA2 decreased, and overexpression of NOX1 significantly exacerbated this abnormality. This confirms that reactive oxygen species can enhance the activity of RyR2 in the sarcoplasmic reticulum while inhibiting the activity of SERCA2, leading to imbalances in intracellular Ca2+ levels. The interaction between alarin and NOX1 can regulate the activity of RyR2 and SERCA2, restoring a new balance. It is worth further investigating whether the interaction between alarin and NOX1 regulates other Ca2+ channels.
Figure 4.
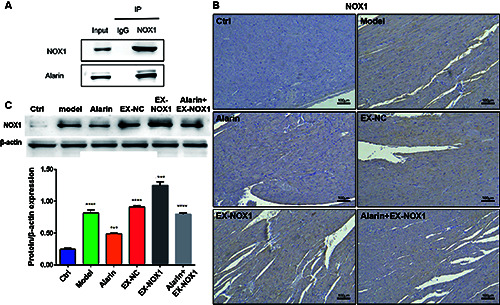
Alarin can interact with NOX1 and suppress the expression of NOX1 in HFpEF rats and H9C2 cells. A) Identification of the interaction between alarin and NOX1 by Co-IP. B) Expression level of NOX1 expression in myocardial tissue; scale bar: 100 μm. C) The expression level of NOX1 in H9C2 cells. The results are expressed as mean ± SEM; n=3 for each group. ***p<0.001; ****p<0.0001.
Figure 5.
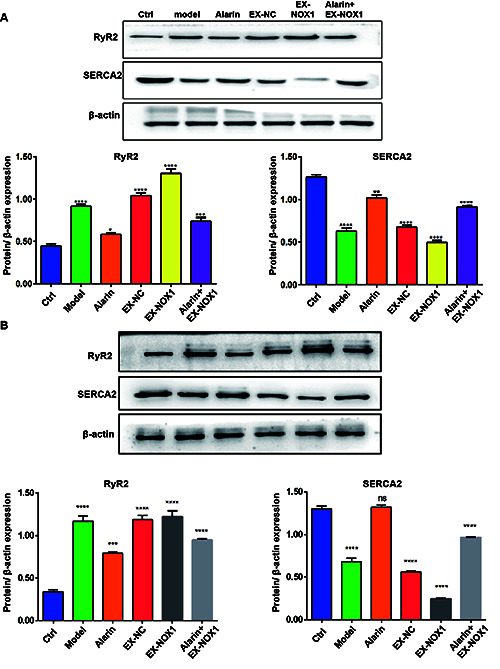
Alarin exhibits the capacity to regulate the expression of RyR2 and SERCA2 in HFpEF. A) Expression level of RyR2 and SERCA2 in myocardial tissue. B) The expression level of RyR2 and SERCA2 in H9C2 cells. The results are expressed as mean ± SEM; n=3 for each group. *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001.
Alarin, first identified in neuroblastomas, is a regulatory peptide consisting of 25 amino acids. Its synthesis begins with transcription of the GALP gene, followed by alternative splicing, which excludes exon 3 of the gene, producing alarin mRNA and propeptide.28 Subsequently, this propeptide is likely to undergo protein hydrolysis and chemical modifications to generate the active form of alarin with 25 amino acids. However, post-translational processing of alarin remains unclear and requires further research.35 Increasing evidence suggests that alarin is involved in various biological functions, including physiological and pathological conditions. In this preliminary study, serum proteomics analysis revealed significantly reduced levels of alarin in HFpEF rats, with similar results detected in cardiac tissue. Further investigation is warranted to understand why alarin expression is suppressed in HFpEF. Based on our previous research, alarin has shown cardioprotective properties and helps prevent cardiac fibrosis during heart failure.27 Studies have demonstrated that alarin can improve cardiac dysfunction and mitigate cardiac fibrosis in HF rats, indicating its efficacy as a cardioprotective agent similar to atrial natriuretic peptide. Alarin reduces cardiac fibrosis in HF rats by lowering the levels of collagen and transforming growth factor-β (TGF-β) induced by Ang II. Additionally, alarin administration to cardiac fibroblasts inhibits fibrosis by attenuating oxidative stress in HF rats induced by MI, acting as an antioxidant.36 Alarin may also alleviate cardiac fibrosis by reversing the elevated Nox1 activity, superoxide anion, and malondialdehyde (MDA) levels, as well as the decreased superoxide dismutase (SOD) levels in MI rats and Ang II-treated cardiac fibroblasts.37 This study further confirms that alarin can bind to NOX1, reducing oxidative stress levels in HFpEF rats, regulating RyR2 and SERCA2 activity, and improving myocardial diastolic function in HFpEF. Due to its cardioprotective effects, alarin holds promise as a potential therapy for HF in the future. However, further extensive research is required to elucidate the pharmacological and physiological mechanisms of alarin in cardiac protection. HFpEF is a type of HF that poses significant challenges for treatment, with no specific therapeutic options currently available. The binding of alarin to NOX1, by improving oxidative stress and regulating Ca2+ homeostasis, may bring new breakthroughs in the treatment of HFpEF. However, current research is still in its preliminary stages, and future studies need to further validate the therapeutic effects, safety, and longterm efficacy of alarin binding to NOX1 in animal models and clinical trials.
When exploring the potential of alarin as a therapeutic agent for HFpEF, we must face its possible side effects and limitations. Although this study has demonstrated the beneficial effects of alarin on cardiac function in cellular and animal models, the translation of these results into clinical applications requires caution. Due to the significant association between alarin and obesity and diabetes, it is attractive as a target for the treatment of these metabolic diseases.28,38,39 However, the safety of alarin in treatment, including potential side effects such as interference with normal physiological metabolic processes or adverse interactions with other drugs, must be carefully evaluated. Furthermore, although alarin may indirectly affect the risk of coronary atherosclerotic heart disease, direct evidence is insufficient, and future studies need to delve deeper into its mechanisms and safety. Finally, due to the heterogeneity of HFpEF, the limitation of alarin lies in its potential inapplicability to all HFpEF patients. Therefore, the therapeutic effect of alarin may vary among patients, necessitating the development of individualized treatment plans. In conclusion, while the clinical application prospects of alarin are broad, its potential risks and limitations must be comprehensively considered.
This study has made preliminary achievements in exploring the cardioprotective mechanisms of alarin in HFpEF, yet there are still some limitations. Firstly, the study has not conducted relevant tests on clinical samples from HFpEF patients, which restricts the direct application of the research conclusions in clinical practice to a certain extent. Secondly, although the possibility of an interaction between alarin and NOX1 has been proposed, the specific molecular mechanisms still require further in-depth research and validation. Furthermore, this study did not cover the long-term efficacy and safety assessment of alarin in HFpEF patients, which represents an important direction for future research. This study provides preliminary clues for the application of alarin in the treatment of HFpEF, but further clinical trials and mechanistic exploration are necessary to verify its effectiveness.
In summary, our study explored the mechanism underlying the protective effects of alarin on the cardiac function in HFpEF. The increasing of oxidative stress level leads to abnormalities in the myocardial tissue structure and Ca2+ channels in HFpEF, ultimately resulting in impaired diastolic function of the heart, which could be attenuated by alarin. The mechanism of these protective effects may partially involve the interaction between alarin and NOX1 leads to the reduction of oxidative stress levels, regulating the activities of RyR2 and SERCA2 to restore the balance of Ca2+ release and absorption, thereby improving the cardiac function in HFpEF. Our findings open new avenues for the treatment of HFpEF.
Funding Statement
Funding: this work was supported by Suqian Sci&Tech Program (Grant No. K202218), Suqian Traditional Chinese Medicine Technology Program (Grant No. HD202214) and Xuzhou Medical University Affiliated Hospital Sci&Tech Program (Grant No. XYFM202325).
Availability of data and materials
The data used to support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599-726. [DOI] [PubMed] [Google Scholar]
- 2.Pagel PS, Tawil JN, Boettcher BT, Izquierdo DA, Lazicki TJ, Crystal GJ, et al. Heart failure with preserved ejection fraction: A comprehensive review and update of diagnosis, pathophysiology, treatment, and perioperative implications. J Cardiothorac Vasc Anesth 2021;35:1839-59. [DOI] [PubMed] [Google Scholar]
- 3.Dal Canto E, Remmelzwaal S, van Ballegooijen AJ, Handoko ML, Heymans S, van Empel V, et al. Diagnostic value of echocardiographic markers for diastolic dysfunction and heart failure with preserved ejection fraction. Heart Fail Rev 2022;27:207-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mishra S, Kass DA. Cellular and molecular pathobiology of heart failure with preserved ejection fraction. Nat Rev Cardiol 2021;18:400-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldstein D, Frishman WH. Diastolic heart failure: a review of current and future treatment options. Cardiol Rev 2021;29:82-8. [DOI] [PubMed] [Google Scholar]
- 6.Sag CM, Santos CX, Shah AM. Redox regulation of cardiac hypertrophy. J Mol Cell Cardiol 2014;73:103-11. [DOI] [PubMed] [Google Scholar]
- 7.Madamanchi NR, Runge MS. Redox signaling in cardiovascular health and disease. Free Radic Biol Med 2013;61:473-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aramouni K, Assaf R, Shaito A, Fardoun M, Al-Asmakh M, Sahebkar A, et al. Biochemical and cellular basis of oxidative stress: Implications for disease onset. J Cell Physiol 2023;238:1951-63. [DOI] [PubMed] [Google Scholar]
- 9.Shaito A, Aramouni K, Assaf R, Parenti A, Orekhov A, Yazbi AE, et al. Oxidative stress-induced endothelial dysfunction in cardiovascular diseases. Front Biosci (Landmark Ed) 2022;27:105. [DOI] [PubMed] [Google Scholar]
- 10.Shi L, Fu W, Xu H, Li S, Yang X, Yang W, et al. Ginsenoside Rc attenuates myocardial ischaemic injury through antioxidative and anti-inflammatory effects. Pharm Biol 2022;60:1038-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buvelot H, Jaquet V, Krause KH. Mammalian NADPH Oxidases. Methods Mol Biol 2019;1982:17-36. [DOI] [PubMed] [Google Scholar]
- 12.Meyer MR, Barton M. GPER blockers as Nox downregulators: A new drug class to target chronic non-communicable diseases. J Steroid Biochem Mol Biol 2018;176:82-7. [DOI] [PubMed] [Google Scholar]
- 13.Barton M, Meyer MR, Prossnitz ER. Nox1 downregulators: A new class of therapeutics. Steroids 2019;152:108494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sag CM, Wagner S, Maier LS. Role of oxidants on calcium and sodium movement in healthy and diseased cardiac myocytes. Free Radic Biol Med 2013;63:338-49. [DOI] [PubMed] [Google Scholar]
- 15.Adachi T, Weisbrod RM, Pimentel DR, Ying J, Sharov VS, Schoneich C, et al. S-Glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nat Med 2004;10:1200-7. [DOI] [PubMed] [Google Scholar]
- 16.Zhang N, Zhu B. The role of the rat sarcoplasmic reticulum Ca2+-ATPase promoter in myocardial ischemia-preconditioning. Mol Cell Biochem 2010;333:311-21. [DOI] [PubMed] [Google Scholar]
- 17.Bovo E, Mazurek SR, Zima AV. The role of RyR2 oxidation in the blunted frequency-dependent facilitation of Ca(2+) transient amplitude in rabbit failing myocytes. Pflugers Arch 2018;470:959-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamilton S, Terentyeva R, Martin B, Perger F, Li J, Stepanov A, et al. Increased RyR2 activity is exacerbated by calcium leak-induced mitochondrial ROS. Basic Res Cardiol 2020;115:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dridi H, Kushnir A, Zalk R, Yuan Q, Melville Z, Marks AR. Intracellular calcium leak in heart failure and atrial fibrillation: a unifying mechanism and therapeutic target. Nat Rev Cardiol 2020;17:732-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spertus JA, Birmingham MC, Nassif M, Damaraju CV, Abbate A, Butler J, et al. The SGLT2 inhibitor canagliflozin in heart failure: the CHIEF-HF remote, patient-centered randomized trial. Nat Med 2022;28:809-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin N, Manoharan K, Davies C, Lumbers RT. Beta-blockers and inhibitors of the renin-angiotensin aldosterone system for chronic heart failure with preserved ejection fraction. Cochrane Db Syst Rev 2021;5:CD12721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lima-Posada I, Stephan Y, Soulie M, Palacios-Ramirez R, Bonnard B, Nicol L, et al. Benefits of the non-steroidal mineralocorticoid receptor antagonist finerenone in metabolic syndrome-related heart failure with preserved ejection fraction. Int J Mol Sci 2023;24:2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mills EG, Izzi-Engbeaya C, Abbara A, Comninos AN, Dhillo WS. Functions of galanin, spexin and kisspeptin in metabolism, mood and behaviour. Nat Rev Endocrinol 2021;17:97-113. [DOI] [PubMed] [Google Scholar]
- 24.Rana T, Behl T, Sehgal A, Singh S, Sharma N, Abdeen A, et al. Exploring the role of neuropeptides in depression and anxiety. Prog Neuropsychopharmacol Biol Psychiatry 2022;114: 110478. [DOI] [PubMed] [Google Scholar]
- 25.Sipkova J, Kramarikova I, Hynie S, Klenerova V. The galanin and galanin receptor subtypes, its regulatory role in the biological and pathological functions. Physiol Res 2017;66:729-40. [DOI] [PubMed] [Google Scholar]
- 26.Brzozowska M, Calka J. Review: occurrence and distribution of galanin in the physiological and inflammatory states in the mammalian gastrointestinal tract. Front Immunol 2020;11: 602070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J, Ding H, Li Y, Zhou H, Wang W, Mei Y, et al. Alarin alleviated cardiac fibrosis via attenuating oxidative stress in heart failure rats. Amino Acids 2021;53:1079-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abebe EC, Mengstie MA, Seid MA, Malik T, Dejenie TA. The evolving roles of alarin in physiological and disease conditions, and its future potential clinical implications. Front Endocrinol (Lausanne) 2022;13:1028982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sorimachi H, Burkhoff D, Verbrugge FH, Omote K, Obokata M, Reddy YNV, et al. Obesity, venous capacitance, and venous compliance in heart failure with preserved ejection fraction. Eur J Heart Fail 2021;23:1648-58. [DOI] [PubMed] [Google Scholar]
- 30.Packer M, Lam CSP, Lund LH, Maurer MS, Borlaug BA. Characterization of the inflammatory-metabolic phenotype of heart failure with a preserved ejection fraction: a hypothesis to explain influence of sex on the evolution and potential treatment of the disease. Eur J Heart Fail 2020;22:1551-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Houstis NE, Eisman AS, Pappagianopoulos PP, Wooster L, Bailey CS, Wagner PD, et al. Exercise intolerance in heart failure with preserved ejection fraction: diagnosing and ranking its causes using personalized O(2) pathway analysis. Circulation 2018;137:148-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monma Y, Shindo T, Eguchi K, Kurosawa R, Kagaya Y, Ikumi Y, et al. Low-intensity pulsed ultrasound ameliorates cardiac diastolic dysfunction in mice: a possible novel therapy for heart failure with preserved left ventricular ejection fraction. Cardiovasc Res 2021;117:1325-38. [DOI] [PubMed] [Google Scholar]
- 33.Triposkiadis F, Xanthopoulos A, Starling RC, Iliodromitis E. Obesity, inflammation, and heart failure: links and misconceptions. Heart Fail Rev 2022;27:407-18. [DOI] [PubMed] [Google Scholar]
- 34.Eisner DA, Caldwell JL, Kistamas K, Trafford AW. Calcium and excitation-contraction coupling in the heart. Circ Res 2017;121:181-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santic R SS, Lang R, Rauch I, Voglas E, Eberhard N, Bauer JW, Brain SD, Kofler B. Alarin is a vasoactive peptide. Proc Natl Acad Sci USA 2007;104:10217-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kura B, Szeiffova Bacova B, Kalocayova B, Sykora M, Slezak J. Oxidative stress-responsive microRNAs in heart injury. Int J Mol Sci 2020;21:358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y, Murugesan P, Huang K, Cai H. NADPH oxidases and oxidase crosstalk in cardiovascular diseases: novel therapeutic targets. Nat Rev Cardiol 2020;17:170-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kilinc F, Demircan F, Gozel N, Onalan E, Karatas A, Pekkolay Z, et al. Assessment of serum alarin levels in patients with type 2 diabetes mellitus. Acta Endocrinol-Buch 2020;16:165-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou X, Luo M, Zhou S, Cheng Z, Chen Z, Yu X. plasma alarin level and its influencing factors in obese newly diagnosed type 2 diabetes patients. Diabet Metab Synd Ob 2021;14:379-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon reasonable request.


