Abstract
Background
In prior observational investigations, it has been demonstrated that the consumption of milk is associated with the incidence of breast cancer (BC). Despite the existence of a two-sample Mendelian randomization (MR) that suggests a causal relationship between milk intake and breast cancer risk, the outcomes still lack a definitive conclusion. This ambiguity may be attributed to variables such as the variety of milk ingested, estrogen levels, the specific type of BC, and potential confounding factors. Therefore, our principal objective is to establish the causal association between the consumption of skimmed milk and full cream milk and the risk of different types of BC through the utilization of two-sample and two-step MR analyses.
Methods
In this study, we analyzed single nucleotide polymorphisms associated with skimmed and full-cream milk consumption in a cohort of 360,806 individuals from European populations through genome-wide association studies. We conducted a two-sample MR analysis using three different methods: inverse variance weighting (IVW) was used as the main analysis, and MR-Egger and Weighted median were used as supplementary analyses to IVW. We also performed sensitivity analysis, which included leave-one-out analysis, Cochran's Q test to detect heterogeneity, and MR-Egger intercept analysis to detect potential biases caused by pleiotropy. We used two-step MR analysis to evaluate potential mediators of associations.
Results
In the two-sample MR analysis, IVW analysis suggests a potential inverse causal relationship between skimmed milk and BC [OR 0.34, 95% CI (0.12–1.00), P = 0.05]. Subgroup analysis revealed that skimmed milk reduces the risk of estrogen receptor-negative (ER−) breast cancer [OR 0.18, 95% CI (0.04–0.90), P = 0.04], but not estrogen receptor-positive (ER+) breast cancer [OR 0.42, 95% CI (0.15–1.22), P = 0.11]. MR Egger reached similar results, that is, skimmed milk reduces the risk of ER− breast cancer [OR 0.006, 95% CI (0.00–0.70), P = 0.04], but not BC [OR 0.16, 95% CI (0.01–4.66), P = 0.30] and ER+ breast cancer [OR 0.50, 95% CI (0.02–12.61), P = 0.65]. Additionally, we found no causal relationship between full cream milk and BC (P > 0.05). In two-step MR analysis, we found evidence for a mediating role of BMI in the relationship between skimmed milk intake and ER-breast cancer risk.
Conclusion
Our findings strengthen the evidence for a protective effect of skimmed milk consumption on ER-breast cancer risk. Further two-step MR analyses suggest that this protective effect may partly result from body mass index (BMI). There is no evidence that full cream milk consumption affects the risk of BC.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12672-024-01448-5.
Keywords: Skimmed milk, Full cream milk, Breast cancer, Mendelian randomization study
Introduction
BC is a worldwide public health concern that primarily affects women [1]. Based on the latest GLOBOCAN data, it has been observed that, among female individuals, BC has now surpassed lung cancer to become the leading cancer in terms of global incidence. In the year 2020, the global incidence of BC led to the diagnosis of more than 2.3 million women, with a regrettable 685,000 fatalities reported as an outcome [1–4]. Recent studies have documented that, beyond hereditary and genetic mutations affecting BC susceptibility [5, 6], acquired dietary and lifestyle factors can exert a substantial influence on the risk of BC [7–9]. Dietary and lifestyle modifications may be effective measures to reduce the risk of breast cancer.
Dairy products constitute a pivotal element of the traditional Western dietary pattern and serve as a prominent reservoir of essential nutrients [10]. The association between milk consumption and cancer risk has been subjected to comprehensive scrutiny. Existing observational evidence indicates that milk consumption is linked to a diminished risk of colorectal cancer [11]. In contrast, alternative observational findings propose a positive effect between milk consumption and the risk of prostate cancer [12]. The results of a substantial body of research on the impact of dairy product consumption on BC risk exhibit notable heterogeneity [13, 14]. Several observational investigations have suggested that the intake of dairy products may decreased the risk of BC. This association is postulated to be related to the presence of various compounds, notably calcium, vitamin D, and lactoferrin, all recognized for their anti-cancer properties, which may contribute to a decreased risk of BC [15, 16]. Conversely, other studies have identified a positive effect between dairy consumption and a heightened susceptibility of BC, which may be attributed to the concomitant increased intake of dietary fat, including saturated fat, elements known to have evidence of effect on augmented risk of BC [17, 18]. In addition, dairy products contain a complex array of contaminants and compounds, including estrogen, implicated in aberrant DNA replication and increased mitotic activity [18]. Furthermore, insulin-like growth factor I (IGF-I) found in milk has been linked to the promotion of BC cell proliferation, consequently amplifying BC risk [14].
Besides that, ambiguity surrounds the influence of different categories of dairy products (such as fermented dairy, high-fat dairy, or low-fat dairy) on BC risk, as well as the effects of these products on different subtypes of BC (e.g., estrogen receptor-positive, progesterone receptor-positive) [17–19]. Moreover, numerous investigations have postulated that dairy products may have evidence of effect on health-related issues, including obesity and diabetes, which may potentially influence BC development [20, 21]. The overall role of dairy products in BC etiology remains elusive, and the causal relationship between milk consumption and BC risk remains indeterminate due to the inherent limitations of observational studies, including confounding and reverse causation.
MR is a causal inference technique that uses genetic variants as instrumental variables to investigate the causal relationship between an exposure and an outcome [22, 23]. MR provides a powerful tool for overcoming confounding and reversing causality issues that can compromise observational studies [24]. Although existing MR analyses have revealed an association between dairy product intake and breast cancer risk, the causal relationship between different dairy products and different types of breast cancer requires further investigation [25, 26]. To provide more scientific preventive strategies for dietary choices related to BC. The aim of this study was to assess the causal association between different dairy product intakes and the risk of developing different types of BC through two-sample MR and analyze the role of possible mediators in this causal relationship.
Method
Data sources
Our study utilized data from the genome-wide association study (GWAS) database (https://gwas.mrcieu.ac.uk/), which is a publicly available database created by the Integrated Epidemiology Unit (IEU) at the University of Bristol [27]. We used MR to investigate the causal relationship between skimmed milk and full cream milk with BC (including ER+ breast cancer and ER− breast cancer). The GWAS data for skimmed milk, full cream milk, and BC (including ER+ breast cancer and ER− breast cancer) were obtained directly or indirectly from the GWAS summary data through the IEU Open GWAS project. Single nucleotide polymorphisms (SNPs) for ER− breast cancer included 21,468 cases and 105,974 controls. Summary-level data for ER+ breast cancer included 69,501 cases and 105,974 controls.
Instrumental variable selection
In the two-sample MR Study, we used SNPs as instrumental variables. When we selected P < 5 × 10–8 as the threshold, there were only a few SNPs had evidence of effect on with skimmed milk and full cream milk, and referring to the criteria of previous articles [28, 29], we choose a more relaxed threshold (P < 5 × 10–6) to get enough SNPs. Additionally, to remove IVs with chained imbalances, we confirmed that these SNPs met the criteria of linkage equilibrium (r2 < 0.001) and clump distance > 10,000 kb [30]. F-statistic > 10 was considered to indicate a strong association between the exposure and outcome variables [31].
Two-sample MR analysis
We selected three commonly used methods to determine the causal relationship between exposure and outcome. These include: (1) IVW: IVW computes the causal impact of an exposure on the outcome by amalgamating the ratio estimates for each SNP, effectively transforming MR estimates into a weighted regression of SNP-outcome effects concerning SNP-exposure effects [32]. (2) Weighted Median: The weighted median method yields unbiased estimates, even when a substantial portion, up to 50%, of the data originates from invalid instrumental variables (IVs) [33]. (3) MR-Egger: the MR-Egger is a tool that provides a causal effect through the slope coefficient from Egger regression and also detects small study bias [34, 35]. Among these methods, IVW was considered the most important for detecting causality [36]. The Cochran's Q test was used to detect heterogeneity. There is heterogeneity when the Cochran's Q test P < 0.05 [37]. The leave-one-out analysis was used to determine whether the causal estimate was driven by any single SNP [38]. In this step, we removed each SNP from the instrumental variables (IVs) one by one and reassessed the causal estimates. If certain SNPs had significant correlations with the exposure, these SNPs might have dominated the causal estimate. Therefore, we should re-estimate the causal effect by excluding all such SNPs to assess robustness.
PhenoScanner GWAS analysis
To further analyze the function and phenotype of each SNP, the PhenoScanner database(http://www.phenoscanner.medschl.cam.ac.uk/) was used to find genes that corresponding to each SNP in skimmed milk.
Two-step MR analysis
We use a two-step MR to analyze potential mediators [39]. (1) Effect of skimmed milk on ER− breast cancer (2) Effect of BMI on ER− breast cancer (3) Regulatory effect of BMI (Fig. 1). The indirect BMI-mediated effect is obtained by multiplying b × c, where b is the MR Effect of skimmed milk on BMI, and c is the MR Effect of mediated BMI on ER-breast cancer, and a is the MR Effect of skimmed milk on ER-breast cancer. we quantified the proportion mediated by dividing indirect effect by the total effect [40, 41]. All analyses were performed using the Two Sample MR package version 0.5.6 in R software.
Fig. 1.
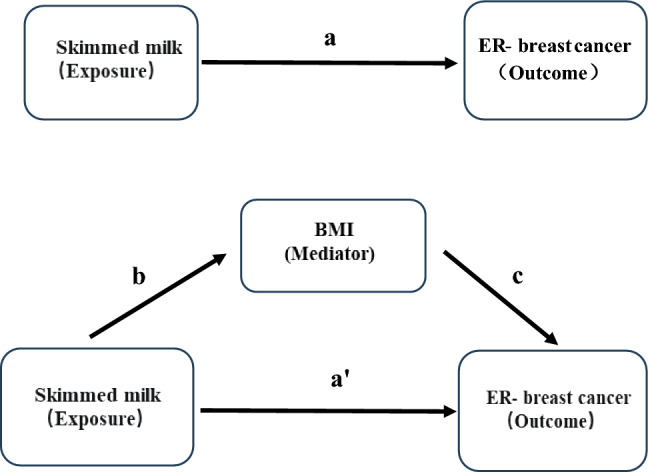
The flow chart of the two-step MR analysis. In mediation analysis where a represents the total effect, a' represents the direct effect and the indirect effect can be calculated by subtracting a' from a (difference method) or multiplying b × c (product of coefficients method). BMI, Body mass index
Result
Exposure and outcome selection
In accordance with the aforementioned criteria for relevance, we have identified 28 SNPs associated with skimmed milk (Supplementary Table 1) and 28 SNPs associated with full cream milk (Supplementary Table 2) as instrumental variables. The statistical F-statistic for full cream milk was calculated to be 232.3, and the corresponding value for skimmed milk was 749.4, suggesting a high degree of instrumental strength. The outcome variables of our study encompassed BC, ER+ breast cancer, and ER− breast cancer. Contributing studies of exposures and outcomes were individuals of European descent The sources were presented in Table 1.
Table 1.
Information of the exposures and outcome datasets
| Information of the exposures and outcome datasets | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GWAS ID | Exposure or outcome | Identified SNPs | Ncase | Ncontrol | Sample size | Number of SNPs | Population | Unit | Author | Consortium | Ontology | Build | F-statistic |
| ukb-d-1418_1 | Full cream milk | 28 | 22,902 | 337,904 | 360,806 | 13,586,587 | European | NA | Neale lab | NA | NA | HG19/GRCh37 | 232.3 |
| ukb-d-1418_3 | Skimmed milk | 28 | 74,087 | 286,719 | 360,806 | 13,586,587 | European | NA | Neale lab | NA | NA | HG19/GRCh37 | 749.4 |
| ieu-a-1126 | Breast cancer | NA | 122,977 | 105,974 | 228,951 | 10,680,257 | European | Log odds | Michailidou K | BCAC | NA | HG19/GRCh37 | NA |
| ieu-a-1127 | ER+ Breast cancer | NA | 69,501 | 105,974 | 175,475 | 10,680,257 | European | Log odds | Michailidou K | BCAC | NA | HG19/GRCh38 | NA |
| ieu-a-1128 | ER− Breast cancer | NA | 21,468 | 105,974 | 127,442 | 10,680,257 | European | Log odds | Michailidou K | BCAC | NA | HG19/GRCh39 | NA |
SNP, Single nucleotide polymorphism. Full cream milk and skimmed milk are exposures. Breast cancer ER+ Breast cancer and ER− Breast cancer are outcomes. F-statistic ≥ 10 suggesting a high degree of instrumental strength
Two-sample MR analysis
IVW analysis indicated a potential inverse association between skimmed milk and BC risk [OR 0.34, 95% CI (0.12–1.00), P = 0.05]. Subgroup analysis revealed that skimmed milk reduces the risk of ER-breast cancer [OR 0.18, 95% CI (0.04–0.90), P = 0.04], but not ER+ breast cancer [OR 0.42, 95% CI (0.15–1.22), P = 0.11]. Furthermore, we found that full cream milk had little evidence of effect on BC risk [P > 0.05]. In addition to the IVW testing method, we also used MR Egger and weighted median methods to verify our results. MR Egger reached similar results, that is, skimmed milk reduces the risk of ER− breast cancer [OR 0.01, 95% CI (0.00–0.70), P = 0.04], but not BC [OR 0.16, 95% CI (0.01–4.66), P = 0.30] and ER+ breast cancer [OR 0.50, 95% CI (0.02–12.61), P = 0.65]. However, weighted median showed that skimmed milk had little evidence of effect on BC (including ER+ breast cancer and ER− breast cancer) risk [P > 0.05]. MR Egger and weighted median showed that full cream milk had little evidence of effect on BC risk (including ER+ breast cancer and ER− breast cancer) [P > 0.05]. The specific MR causal relationships are presented in Table 2.
Table 2.
The results of MR analyses
| The results of Mendelian randomization analyses | |||||||
|---|---|---|---|---|---|---|---|
| Exposure | Outcome | Method | |||||
| Inverse variance weighted | Weighted median | MR Egger | |||||
| OR95%CI | P-value | OR95%CI | P-value | OR95%CI | P-value | ||
| Skimmed milk (ukb-d-1418_3) (Ns:74087) | ER− Breast cancer (ieu-a-1128) (Ns:21468) | 0.18 (0.04–0.90) | 0.04 | 0.26 (0.06–1.11) | 0.07 | 0.01 (0.00–0.70) | 0.04 |
| ER+ Breast cancer (ieu-a-1127) (Ns:69501) | 0.42 (0.15–1.22) | 0.11 | 1.32 (0.52–3.36) | 0.55 | 0.46 (0.02–12.61) | 0.65 | |
| Breast cancer (ieu-a-1126) (Ns:122977) | 0.34 (0.12–1.00) | 0.05 | 0.68 (0.31–1.49) | 0.36 | 0.16 (0.01–4.66) | 0.30 | |
| Full cream milk (ukb-d-1418_1) (Ns:22902) | ER− Breast cancer (ieu-a-1128) (Ns:21468) | 7.58 (0.96–59.59) | 0.05 | 3.16 (0.34–29.85) | 0.31 | 10.98 (0.02–7112.67) | 0.48 |
| ER+ Breast cancer (ieu-a-1127) (Ns:69501) | 2.44 (0.52− 11.45) | 0.26 | 1.19 (0.26–5.51) | 0.83 | 1.04 (0.01–122.70) | 0.99 | |
| Breast cancer (ieu-a-1126) (Ns:122977) | 2.88 (0.64–12.88) | 0.17 | 1.49 (0.45–5.01) | 0.52 | 2.21 (0.02–234.56) | 0.74 | |
MR, Mendelian randomization; ER−, Estrogen receptor-negative; ER+, Estrogen receptor-positive; CI, Confidence interval; OR, Odds ratio
Heterogeneity and pleiotropy test
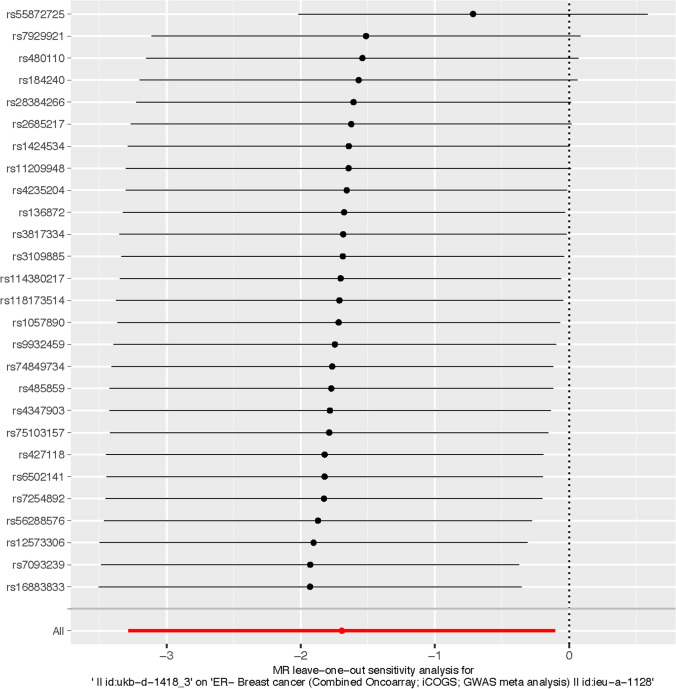
The Cochran's Q test analysis indicated heterogeneity between skimmed milk and full cream milk regarding their effect with BC (including ER+ breast cancer and ER− breast cancer) (P < 0.05). However, the MR-Egger intercept analysis showed that the evidence of horizontal pleiotropy is limited between skimmed milk, full cream milk, and BC (including ER+ breast cancer and ER− breast cancer) (P > 0.05) (Table 3). The leave-one-out analysis revealed that rs55872725 in skimmed milk plays an important role of the results (Fig. 2).
Table 3.
The tests for heterogeneity and horizontal pleiotropy
| Exposure | Outcome | Cochrane’s Q test | Pleiotropy | |||
|---|---|---|---|---|---|---|
| Q | P-value | MR egger intercept | SE | P-value | ||
| Skimmed milk (ukb-d-1418_3) | ER− Breast cancer (ieu-a-1128) | 81.40 | 1.27E−07 | 2.20E−02 | 0.02 | 0.15 |
| ER+ Breast cancer (ieu-a-1127) | 85.22 | 3.22E−08 | −1.00E−03 | 0.01 | 0.96 | |
| Breast cancer (ieu-a-1126) | 124.53 | 7.95E-15 | 5.00E−03 | 0.01 | 0.64 | |
| Full cream milk (ukb-d-1418_1) | ER− Breast cancer (ieu-a-1128) | 44.73 | 1.26E−02 | −1.00E−03 | 0.01 | 0.91 |
| ER+ Breast cancer (ieu-a-1127) | 59.19 | 2.15E−04 | 3.00E−03 | 0.01 | 0.71 | |
| Breast cancer (ieu-a-1126) | 78.70 | 3.32E−07 | 1.00E−03 | 0.01 | 0.91 | |
MR, Mendelian randomization; SE, Standard Error; ER−, Estrogen receptor-negative; ER+, Estrogen receptor-positive
Fig. 2.
The Leave-one-out analysis of skimmed milk and ER− breast cancer
PhenoScanner GWAS analysis
Using the PhenoScanner database, we analyzed 28 SNPs of skimmed milk associated with ER-breast cancer and analyze the gene corresponding to each SNP. Of note, five SNPs (Table 4) were associated with BMI.
Table 4.
The 5 SNPs associated with BMI
| 5 of 27 SNPS in skimmed milk were associated with BMI | ||||
|---|---|---|---|---|
| SNP | Consequence | Ensembl | Hgnc | |
| 1 | rs7254892 | Intron | – | NECTIN2 |
| 2 | rs485859 | Downstream | – | RP11-795H16.3 |
| 3 | rs118173514 | Intron | ENSG00000224078 | SNHG14 |
| 4 | rs3817334 | Intron | ENSG00000109919 | MTCH2 |
| 5 | rs55872725 | Intron | ENSG00000140718 | FTO |
Two-step MR analysis of the role of BMI in the causal relationship between skimmed milk and ER-breast cancer
To further investigate the role of BMI in the reduction of ER-breast cancer risk with skimmed milk, we performed two-step MR analysis. IVW analysis indicated a positive effect between skimmed milk and BMI [OR 5.18, 95% CI (1.87–14.36), P = 2.00E−03], and there is an inverse effect between BMI and ER-breast cancer [OR 0.82, 95% CI (0.72–0.93), P = 2.00E-03] (Table 5). By the two-step MR analysis, it was found that the proportion mediated by BMI was 15.86% (Table 6).
Table 5.
The result of two-step MR analysis BMI as a mediating factor between skimmed milk and ER-breast cancer
| Method | |||||||
|---|---|---|---|---|---|---|---|
| Inverse variance weighted | Weighted median | MR Egger | |||||
| OR95%CI | P-value | OR95%CI | P-value | OR95%CI | P-value | ||
| Exposure | Mediator | 5.18 (1.87–14.36) | 2.00E−03 | 1.27 (0.89–1.81) | 0.19 | 6.77 (0.26–172.84) | 0.26 |
| Skimmed milk (ukb-d-1418_3) | BMI (ukb-a-248) | ||||||
| Mediator | Outcome | 0.82 (0.72–0.93) | 2.00E−03 | 0.86 (0.74–1.00) | 0.04 | 0.40 (0.28–0.56) | 9.61 |
| BMI (ukb-a-248) | ER− Breast cancer (ieu-a-1128) | ||||||
| Exposure | Outcome | 0.99 (0.99–1.00) | 0.07 | 1.00 (0.99–1.00) | 0.09 | 1.00 (0.98–1.01) | 0.66 |
| ER− Breast cancer (ieu-a-1128) | Skimmed milk (ukb-d-1418_3) | ||||||
ER−, Estrogen receptor-negative; CI, Confidence interval; OR, Odds ratio
Table 6.
The result of mediation MR analysis
| Exposure | Outcome | Mediator | b | c | a | a' | Mediation Effect(%) |
|---|---|---|---|---|---|---|---|
| Skimmed milk (ukb-d-1418_3) | ER− Breast cancer (ieu-a-1128) | BMI | 1.64 | -0.2 | -1.69 | -1.36 | 15.86% |
In mediation analysis where a represents the total effect, a' represents the direct effect and the indirect effect can be calculated by subtracting a’ from a (difference method) or multiplying b × c (product of coefficients method).
Discussion
In the context of this MR and potential mediator analysis, we scrutinized the causal relationship between milk consumption and the susceptibility to BC. Our findings validated a protective effect between the consumption of skimmed milk and a diminished risk of ER− breast cancer. Additionally, we uncovered no compelling evidence to substantiate an impact of full cream milk consumption on the risk of BC. Further mediation analyses elucidated that consumption of skimmed milk may reduce ER-breast cancer risk partly through BMI.
The question of whether dairy products exert a protective or detrimental influence on the development of BC in women is a subject of ongoing debate. Overall, existing epidemiological research has yielded inconclusive findings concerning the association between dairy consumption and the incidence of BC. While certain constituents of milk are positively linked to an increased risk of BC [42, 43], others seem to have a protective effect [13]. Dairy products are rich in calcium, vitamin D and conjugated linoleic acid. These substances affect cell proliferation and differentiation and inhibit tumor development [44]. In contrast, the high levels of fat, saturated fat, and potentially carcinogenic contaminants such as pesticides, estrogen metabolites, and growth factors (e.g., IGF-1) contained in dairy products are also supported by prospective cohort studies that suggest an increased risk of ER+ breast cancer with milk consumption [45].
Acknowledging the pivotal influence of estrogen receptors and lipid metabolism on BC susceptibility, our study is primarily concerned with investigating the causal relationships between the consumption of full cream milk and skimmed milk and the incidence of ER− breast cancer and ER+ breast cancer. The meta-analyses of several previous observational studies have suggested an association between the consumption of milk and dairy products, especially low-fat or fermented dairy products, and a reduced risk of BC [44, 46, 47]. Our study provides further support for this notion by indicating that skimmed milk intake decreases the risk of ER-breast cancer, aligning with prior observational research [43, 47]. We hypothesize that the reason behind this risk reduction may be attributed to the relatively low saturated fat content in skimmed milk and the possibility that individuals with ER− breast cancer may exhibit lower sensitivity to estrogen-related substances found in milk. Evidence suggests that the potentially saturated fat and estrogen in full cream milk increases the risk of breast cancer, while the protective molecules in skimmed milk inhibit the development of breast cancer, which may ultimately be the reason we find that skimmed milk reduces the risk of ER breast cancer.
BMI was found to have an important role in our analysis of potential mediators of skimmed milk in reducing the incidence of ER− breast cancer. It has been reported that there is an inverse relationship between obesity and the risk of premenopausal BC [48, 49]. Seven prospective cohort studies, encompassing 337,819 women and 4,385 cases of invasive BC, reported an inverse association between BMI and the risk of BC [50]. A meta-analysis also indicated that BMI has evidence of effect on a reduced risk of BC incidence [51]. Furthermore, other observational studies have reported an inverse relationship between obesity and the risk of postmenopausal ER− breast cancer and TNBC (triple-negative breast cancer). A breast X-ray cohort study conducted in Sweden involving 51,823 postmenopausal women found an inverse association between obesity and hormone receptor-negative BC [52]. This relationship has also been observed in other studies [53–55]. Rs55872725 was located in the intron of the FTO gene(https://www.ncbi.nlm.nih.gov/snp), which had a very strong correlation with BMI [56]. Several previous studies have shown an association between obesity and breast cancer incidence [57, 58], suggesting that BMI may be associated with a causal relationship between skimmd milk and ER-breast cancer. In our analysis of genes associated with SNPs related to skimmed milk, we found that some of these genes were strongly associated with obesity, suggesting that BMI may play a role in the reduction of ER-breast cancer with skimmed milk. We then further confirmed the above hypothesis in mediating factor analysis, indicating that skimmed milk partially reduces the risk of breast cancer through BMI.
Our study has several limitations that should be taken into consideration. Firstly, the use of GWAS data has inherent limitations. For instance, we were unable to evaluate the non-linear relationship between milk consumption and BC risk or assess BC risk at different ages [59]. Secondly, since the number of instrumental variables was insufficient for analysis when using a genome-wide significance threshold, we opted for a P < 5e−06 threshold. This threshold was also based on other literature. Moreover, our Mendelian randomization and pleiotropy results indicate the relative reliability of our findings. However, it is still not possible to completely rule out the potential "contamination" of the genetic instruments for the exposure of interest by these variants, and further analysis is needed. Thirdly, in our mediation analysis, although the primary IVW analysis suggests that BMI may have a potential mediating role in the causal relationship between skimmed milk and the risk of ER-breast cancer, the P-values for the weighted median and MR Egger tests were not statistically significant, requiring further validation with external data. Additionally, the unclear timing of data collection in existing databases means we cannot accurately test mediation behaviors. Moreover, as we analyzed our exposure phenotypes at the genetic level, our results may not be directly comparable to those of traditional observational studies. Additionally, our analysis was limited to European populations, and it is unclear whether the observed association can be extrapolated to other populations.
In summary, we revealed skimmed milk reduces the risk of ER− breast cancer by the MR study. In contrast, there was no significant causal relationship between full cream milk and BC, including both ER+ breast cancer and ER− breast cancer. Further the two-step MR analyses suggest that this protective effect may partly result from BMI. Overall, increasing the intake of skimmed milk could be a viable strategy for preventing ER− breast cancer.
Supplementary Information
Acknowledgements
The authors would like to express their gratitude to the participants of all the consortia mentioned in this study for generously sharing their data and making it publicly available.
Abbreviations
- BC
Breast cancer
- BMI
Body mass index
- ER−
Estrogen receptor-negative
- ER+
Estrogen receptor-positive
- CI
Confidence interval
- GWAS
Genome-wide association study
- IVs
Tool variable
- IVW
Inverse-variance-weighted
- MR
Mendelian randomization
- OR
Odds ratio
- SNP
Single nucleotide polymorphism
- SE
Standard error
Author contributions
Huijing Wu and Chang Xue conceptualized the study. Yingdan Huang and Wangjin Zhang conducted the literature search and drafted the manuscript with Wangjin Zhang. Jinghui Chen and Sihua Qiu conducted the statistical analyses. Yingdan Huang provided critical revisions to the manuscript for important intellectual content. All authors read and approved the final version of the manuscript.
Funding
This research supported by Beijing Xisike Clinical Oncology Research Foundation.
Data availability
Publicly available datasets were analyzed in this study. This data can be found here: 1. https://gwas.mrcieu.ac.uk/. 2. https://www.ncbi.nlm.nih.gov/snp. 3. http://www.phenoscanner.medschl.cam.ac.uk/. Requests for this information can be directed to the provider.
Declarations
Ethics approval and consent to participate
In this study, publicly available data sets without any personal information were analyzed. There was no need for extra ethical approval and consent statement because the initial GWAS already had the approval of the appropriate ethics review committees and patient consent.
Guarantor
Huijing Wu was the guarantor.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yingdan Huang and Wangjin Zhang contributed equally to this work.
Contributor Information
Chang Xue, Email: xuechang91@163.com.
Huijing Wu, Email: whjky122634@163.com.
References
- 1.Siegel RL, et al. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. [DOI] [PubMed] [Google Scholar]
- 2.Global Burden of Disease Cancer, C. et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol. 2019;5(12):1749–68. [DOI] [PMC free article] [PubMed]
- 3.Giaquinto AN, et al. Breast cancer statistics, 2022. CA Cancer J Clin. 2022;72(6):524–41. [DOI] [PubMed] [Google Scholar]
- 4.Sung H, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. [DOI] [PubMed] [Google Scholar]
- 5.Lee A, Moon BI, Kim TH. BRCA1/BRCA2 pathogenic variant breast cancer: treatment and prevention strategies. Ann Lab Med. 2020;40(2):114–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwong A, et al. Comprehensive spectrum of BRCA1 and BRCA2 deleterious mutations in breast cancer in Asian countries. J Med Genet. 2016;53(1):15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Britt KL, Cuzick J, Phillips KA. Key steps for effective breast cancer prevention. Nat Rev Cancer. 2020;20(8):417–36. [DOI] [PubMed] [Google Scholar]
- 8.Winters S, et al. Breast cancer epidemiology, prevention, and screening. Prog Mol Biol Transl Sci. 2017;151:1–32. [DOI] [PubMed] [Google Scholar]
- 9.Pashayan N, et al. Personalized early detection and prevention of breast cancer: ENVISION consensus statement. Nat Rev Clin Oncol. 2020;17(11):687–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willett WC, Ludwig DS. Milk and health. N Engl J Med. 2020;382(7):644–54. [DOI] [PubMed] [Google Scholar]
- 11.Barrubes L, et al. Association between dairy product consumption and colorectal cancer risk in adults: a systematic review and meta-analysis of epidemiologic studies. Adv Nutr. 2019;10(suppl_2):S190–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lopez-Plaza B, et al. Milk and dairy product consumption and prostate cancer risk and mortality: an overview of systematic reviews and meta-analyses. Adv Nutr. 2019;10(suppl_2):S212–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arafat HM, et al. The association between breast cancer and consumption of dairy products: a systematic review. Ann Med. 2023;55(1):2198256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zang J, et al. The association between dairy intake and breast cancer in Western and Asian populations: a systematic review and meta-analysis. J Breast Cancer. 2015;18(4):313–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hjartaker A, et al. Dairy consumption and calcium intake and risk of breast cancer in a prospective cohort: the Norwegian Women and Cancer study. Cancer Causes Control. 2010;21(11):1875–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kesse-Guyot E, et al. Dairy products, calcium and the risk of breast cancer: results of the French SU.VI.MAX prospective study. Ann Nutr Metab. 2007;51(2):139–45. [DOI] [PubMed] [Google Scholar]
- 17.Ronco AL, De Stefani E, Dattoli R. Dairy foods and risk of breast cancer: a case-control study in Montevideo. Uruguay Eur J Cancer Prev. 2002;11(5):457–63. [DOI] [PubMed] [Google Scholar]
- 18.Kaluza J, et al. Long-term consumption of non-fermented and fermented dairy products and risk of breast cancer by estrogen receptor status—population-based prospective cohort study. Clin Nutr. 2021;40(4):1966–73. [DOI] [PubMed] [Google Scholar]
- 19.He Y, et al. The relationship between dairy products intake and breast cancer incidence: a meta-analysis of observational studies. BMC Cancer. 2021;21(1):1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Sullivan TA, et al. Dairy product consumption, dietary nutrient and energy density and associations with obesity in Australian adolescents. J Hum Nutr Diet. 2015;28(5):452–64. [DOI] [PubMed] [Google Scholar]
- 21.Zong G, et al. Dairy consumption, type 2 diabetes, and changes in cardiometabolic traits: a prospective cohort study of middle-aged and older Chinese in Beijing and Shanghai. Diabetes Care. 2014;37(1):56–63. [DOI] [PubMed] [Google Scholar]
- 22.Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. 2014;23(R1):R89-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burgess S, et al. A robust and efficient method for Mendelian randomization with hundreds of genetic variants. Nat Commun. 2020;11(1):376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith GD, et al. Clustered environments and randomized genes: a fundamental distinction between conventional and genetic epidemiology. PLoS Med. 2007;4(12):e352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larsson SC, et al. Genetically proxied milk consumption and risk of colorectal, bladder, breast, and prostate cancer: a two-sample Mendelian randomization study. BMC Med. 2020;18(1):370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lumsden AL, Mulugeta A, Hypponen E. Milk consumption and risk of twelve cancers: a large-scale observational and Mendelian randomisation study. Clin Nutr. 2023;42(1):1–8. [DOI] [PubMed] [Google Scholar]
- 27.Yang W, et al. Dietary factors and risk for asthma: a Mendelian randomization analysis. Front Immunol. 2023;14:1126457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He H, et al. Causal relationships between metabolic-associated fatty liver disease and iron status: two-sample Mendelian randomization. Liver Int. 2022;42(12):2759–68. [DOI] [PubMed] [Google Scholar]
- 29.Choi KW, et al. Assessment of bidirectional relationships between physical activity and depression among adults: a 2-sample Mendelian randomization study. JAMA Psychiat. 2019;76(4):399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen X, et al. Kidney damage causally affects the brain cortical structure: a Mendelian randomization study. EBioMedicine. 2021;72:103592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bennett DA. An introduction to instrumental variables analysis: part 1. Neuroepidemiology. 2010;35(3):237–40. [DOI] [PubMed] [Google Scholar]
- 32.Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. 2013;37(7):658–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bowden J, et al. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40(4):304–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Egger M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hartwig FP, et al. Inflammatory biomarkers and risk of schizophrenia: A 2-sample Mendelian randomization study. JAMA Psychiat. 2017;74(12):1226–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bowden J, Holmes MV. Meta-analysis and Mendelian randomization: a review. Res Synth Methods. 2019;10(4):486–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen X, et al. Causal relationship between physical activity, leisure sedentary behaviors and COVID-19 risk: a Mendelian randomization study. J Transl Med. 2022;20(1):216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carter AR, et al. Mendelian randomisation for mediation analysis: current methods and challenges for implementation. Eur J Epidemiol. 2021;36(5):465–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao SS, et al. The impact of education inequality on rheumatoid arthritis risk is mediated by smoking and body mass index: Mendelian randomization study. Rheumatology (Oxford). 2022;61(5):2167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen J, et al. Role of inflammatory factors in mediating the effect of lipids on nonalcoholic fatty liver disease: a two-step, multivariable Mendelian randomization study. Nutrients. 2022;14(20):4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kazemi A, et al. Intake of various food groups and risk of breast cancer: a systematic review and dose-response meta-analysis of prospective studies. Adv Nutr. 2021;12(3):809–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fraser GE, et al. Dairy, soy, and risk of breast cancer: those confounded milks. Int J Epidemiol. 2020;49(5):1526–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu Y, et al. Dairy foods, calcium, and risk of breast cancer overall and for subtypes defined by estrogen receptor status: a pooled analysis of 21 cohort studies. Am J Clin Nutr. 2021;114(2):450–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parodi PW. Dairy product consumption and the risk of breast cancer. J Am Coll Nutr. 2005;24(6 Suppl):556S-S568. [DOI] [PubMed] [Google Scholar]
- 46.Dong JY, et al. Dairy consumption and risk of breast cancer: a meta-analysis of prospective cohort studies. Breast Cancer Res Treat. 2011;127(1):23–31. [DOI] [PubMed] [Google Scholar]
- 47.Wu J, et al. Dietary protein sources and incidence of breast cancer: a dose-response meta-analysis of prospective studies. Nutrients. 2016;8(11):730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.White AJ, et al. Overall and central adiposity and breast cancer risk in the Sister Study. Cancer. 2015;121(20):3700–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Michels KB, Terry KL, Willett WC. Longitudinal study on the role of body size in premenopausal breast cancer. Arch Intern Med. 2006;166(21):2395–402. [DOI] [PubMed] [Google Scholar]
- 50.van den Brandt PA, et al. Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am J Epidemiol. 2000;152(6):514–27. [DOI] [PubMed] [Google Scholar]
- 51.Bergstrom A, et al. Overweight as an avoidable cause of cancer in Europe. Int J Cancer. 2001;91(3):421–30. [DOI] [PubMed] [Google Scholar]
- 52.Suzuki R, et al. Body weight and postmenopausal breast cancer risk defined by estrogen and progesterone receptor status among Swedish women: a prospective cohort study. Int J Cancer. 2006;119(7):1683–9. [DOI] [PubMed] [Google Scholar]
- 53.Phipps AI, et al. Body size, physical activity, and risk of triple-negative and estrogen receptor-positive breast cancer. Cancer Epidemiol Biomarkers Prev. 2011;20(3):454–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Neuhouser ML, et al. Overweight, obesity, and postmenopausal invasive breast cancer risk: a secondary analysis of the women’s health initiative randomized clinical trials. JAMA Oncol. 2015;1(5):611–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li CI, Malone KE, Daling JR. Interactions between body mass index and hormone therapy and postmenopausal breast cancer risk (United States). Cancer Causes Control. 2006;17(5):695–703. [DOI] [PubMed] [Google Scholar]
- 56.Lindstrom S, et al. A comprehensive survey of genetic variation in 20,691 subjects from four large cohorts. PLoS ONE. 2017;12(3):e0173997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Picon-Ruiz M, et al. Obesity and adverse breast cancer risk and outcome: mechanistic insights and strategies for intervention. CA Cancer J Clin. 2017;67(5):378–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fortner RT, et al. Obesity and breast cancer. Recent Results Cancer Res. 2016;208:43–65. [DOI] [PubMed] [Google Scholar]
- 59.Carwile JL, Willett WC, Michels KB. Consumption of low-fat dairy products may delay natural menopause. J Nutr. 2013;143(10):1642–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: 1. https://gwas.mrcieu.ac.uk/. 2. https://www.ncbi.nlm.nih.gov/snp. 3. http://www.phenoscanner.medschl.cam.ac.uk/. Requests for this information can be directed to the provider.