Abstract
Background
Tuberculous pleural effusion (TPE) is the most common form of extrapulmonary tuberculosis in many settings. The diagnostic performance of the frontline polymerase chain reaction–based GeneXpert MTB/RIF Ultra (Xpert Ultra) remains suboptimal (sensitivity of ∼30%), but data are limited. Improved diagnostic approaches are urgently needed to detect extrapulmonary tuberculosis (EPTB) in tuberculosis (TB)-endemic settings.
Methods
This multicenter, prospective cohort study evaluated the diagnostic performance of a rapid (same-day) interferon gamma rapid immunosuspension assay (IRISA-TB) in patients with presumed TPE from South Africa and India. Participants underwent pleural biopsy, and testing with other available same-day diagnostic assays (adenosine deaminase [ADA], Xpert Ultra, and IRISA-TB) was concurrently undertaken. The reference standard for TB was microbiological and/or histopathological confirmation using pleural fluid and/or pleural biopsy samples.
Results
A total of 217 participants with presumed TPE were recruited (106 from South Africa, 111 from India). The sensitivity of IRISA-TB (cut-point 20.5 pg/mL) was significantly better than that of Xpert Ultra (81.8% [70.4–90.2] vs 32.9% [22.1–45.1]; P < .001) and ADA at the 40 IU/mL cut-point used in India (81.8% [70.4–90.2] vs 53.8% [41.0–66.3]; P = .002). Compared with ADA at the 30 IU/mL cut-point used in South Africa, IRISA-TB had a higher specificity (96.6% [90.3–99.3] vs 87.1% [78.6–93.2]) and a higher positive predictive value (94.7% [85.5–97.3] vs 81.8% [72.4–88.5]). The negative predictive value (NPV; rule-out value) of IRISA-TB was significantly better than that of Xpert Ultra (87.5% [83.2–93.0] vs 64.9% [61.1–68.6]; P < .001) and ADA at the 40 IU/mL cut-point (87.5% [83.2–93.0] vs 74.1% [68.7–79.0]; P < .001).
Conclusions
IRISA-TB demonstrated markedly better sensitivity and NPV than Xpert Ultra and excellent specificity for the diagnosis of TPE. These data have implications for clinical practice in TB-endemic settings.
Keywords: diagnostic accuracy, diagnostics, Mycobacterium tuberculosis, tuberculous pleural effusion, unstimulated IFN-γ
A new low-cost same-day test for extrapulmonary tuberculosis, IRISA-TBTM, outperformed nested PCR (Xpert Ultra) and ADA for the diagnosis of pleural tuberculosis in a prospective study in India and South Africa.
Tuberculosis (TB) is the most common fatal infectious disease globally, now annually surpassing deaths due to coronavirus disease 2019 (COVID–19) [1, 2]. In 2022, there were an estimated 10.6 million newly diagnosed individuals with TB and ∼1.3 million deaths [1]. A significant minority of these (∼20%) were due to extrapulmonary TB (EPTB; ∼2 million newly diagnosed persons). However, EPTB accounts for almost a third of all TB in many HIV-endemic settings [3]. In several endemic settings, the most common manifestation of EPTB is TB pleural effusion (TPE) [3, 4]. The differential diagnosis is wide and may include acute lower respiratory tract infection, malignancy, left ventricular failure, chronic renal failure, pulmonary embolism, autoimmune disease, and drug reactions. For the diagnosis of TPE, a pleural fluid sample is usually obtained by needle aspiration (sputum is usually not obtainable or negative). However, making the diagnosis is problematic, and the current conventional diagnostic tools perform suboptimally in TPE (eg, smear microscopy and culture have sensitivities of ∼2% and 40%, respectively), and results of culture are delayed for several weeks. TB antigen detection (lipoarabinomannan [LAM]) in pleural and pericardial effusion has a sensitivity of <20% [5, 6].
The diagnostic performance of frontline nucleic acid amplification tests (NAATs), such as the GeneXpert, for TPE has been poor. An earlier version (GeneXpert MTB/RIF) had a sensitivity of ∼30% using pleural biopsy as a reference standard and ∼50% using fluid culture, which tends to overestimate performance [7]. More recently, a new ultra-sensitive cartridge was developed (GeneXpert MTB/RIF Ultra [Xpert Ultra]) with ∼15% better sensitivity in sputum smear-negative TB [8, 9]. However, there are limited data using Xpert Ultra for the diagnosis of TPE [10]. Indeed, the results are based on only 49 persons with definite TB pleural effusion and from a single center from one country; thus, external validation data are needed. Histopathological analysis and culture of pleural biopsy material have an excellent yield, but biopsy sampling is often not performed due to resource constraints given the overburden in TB-endemic countries.
Given these shortcomings and the paucibacillary nature of TPE, an immunodiagnostic approach has traditionally been used by measuring adenosine deaminase (ADA; an enzyme produced by mononuclear cells) levels in pleural fluid [11]. However, there are several challenges and hurdles with this approach. First, specificity has been shown to be suboptimal (60%–70%) in several studies when using pleural biopsy as a reference standard, and the negative predictive value (rule-out value) was also suboptimal at ∼70%, meaning that it was a poor tool to portend an alternative diagnosis, hence the need for pleural biopsy [7, 10]. Second, different ADA cut-points have been used in different parts of the world (eg, South Africa uses the 30 IU/mL cut-point, whereas the 40 IU/mL cut-point is used in India, which improves specificity, but sensitivity is considerably lower at ∼50%) [7].
More recently, and to circumvent these shortcomings, a same-day clinically validated single/multiple-use assay to detect unstimulated interferon gamma (IFN-γ) in pleural fluid was developed. This assay (IRISA–TB) showed excellent sensitivity of ∼90%–95% for the diagnosis of TPE (and other forms of TB serositis) and a specificity of ∼95% [5, 7, 10, 12]. Prior studies, including systematic reviews and meta-analyses, have also shown that unstimulated IFN-γ is an excellent diagnostic test for TPE [13–15], but until now a standardized clinically validated assay has been unavailable. It is important to note that IRISA–TB uses unprocessed and unstimulated de novo pleural fluid and must be distinguished from interferon-gamma release assays (IGRAs; eg, QuantiFERON-TB Gold In-Tube and T–SPOT.TB), which require overnight stimulation and co-culture with TB antigens. Thus, IRISA–TB is not an IGRA [16]. However, it uses bespoke and clinically validated antibody pairs that have better sensitivity in TB serositis, which presents its own specific technical challenges.
Given the limited data on the performance of IRISA–TB vs Xpert Ultra (49 patients with confirmed TB) in a single setting, we undertook a multicenter prospective cohort study with IRISA–TB in patients with presumed TPE from two different countries (with differing strain profiles, one with a high and the other with a low HIV burden) using pleural biopsy and microbiology as a composite reference standard.
METHODS
Study Design and Patient Recruitment
We conducted a multicenter, prospective cohort study at Groote Schuur Hospital, Cape Town, South Africa, and Christian Medical Centre, Vellore, India, from February 2019 to June 2022. Patients aged ≥18 years who were willing and able to provide written informed consent and who presented with presumed TPE (any TB symptoms, including cough, fever, night sweats, loss of weight, hemoptysis, chest pain, and/or shortness of breath, as well as a chest x-ray [CXR] showing features of a pleural effusion) were prospectively recruited and included in the study. Patients who were unable to provide written informed consent, had a history of substance or alcohol abuse, or who were pregnant or breastfeeding were excluded from the study.
Sample Collection and Routine Laboratory Tests
Pleural fluid was collected by ultrasound-guided pleurocentesis. Pleural fluid samples were subjected to routine biochemical and cytological analyses in accordance with local standard of care. This included measurement of total protein, albumin, glucose, cytology, ADA, Xpert Ultra, and liquid culture for M. tuberculosis using the MGIT 960 (Becton, Dickinson, Sparks, MD, USA). As it is not considered the standard of care in many TB-endemic countries, as was the case at our institution, differential cell counts (lymphocyte count) were not available. Pleural fluid ADA levels of >30 IU/mL and >40 IU/mL were reported as suggestive of pleural TB in accordance with national guidelines in South Africa and India, respectively [7, 17]. The remaining fluid was placed in a biobank, frozen at −80°C, and subsequently used for IRISA-TB testing (measuring unstimulated IFN-γ). To aid in achieving a final diagnosis for the patient, all individuals who had a non-diagnostic pleurocentesis (eg, if pleural fluid Xpert or cytological analysis was negative for TB or malignancy, respectively) underwent either a “closed” (Abrams Needle) or thoracoscopic pleural biopsy according to routine practice and expertise at the local center. Pleural biopsy samples were sent to local laboratories for histology, Xpert Ultra, and liquid TB culture as part of routine care. Sputum was also concurrently collected for routine smear microscopy and liquid TB culture. HIV testing was performed in all consenting patients.
Study Definitions and Patient Categorization
Due to the limitations imposed by the lack of a “perfect” gold standard for the diagnosis of TPE, a composite reference standard was used for patient categorization (this reference standard was used in all analyses presented). Patients were categorized as (i) definite TPE: patients with a microbiological test specific to M. tuberculosis (Xpert Ultra and/or TB culture positivity in pleural fluid, biopsy specimen, and/or concurrent sputum) and/or the presence of caseating or necrotizing granulomatous inflammation with acid-fast bacilli (AFB) suggestive of TB on histological examination of pleural biopsy tissue and with improvement on anti-TB treatment (all patients in this category received anti-TB treatment); (ii) probable TPE: patients not meeting the criteria for definite TPE but with clinical and radiological indicators suggestive of TB and who were initiated on and responded to anti-TB treatment (all patients in this category received a complete course of anti-TB treatment); (iii) non-TPE: patients with no microbiological or histological evidence of M. tuberculosis and/or for whom an alternative diagnosis was available (these patients did not receive anti-TB treatment either at presentation or on follow-up); and (iv) unclassifiable: patients who could not be subjected to the composite reference standard, were lost to follow-up, or died before the assessment of TB treatment response or obtaining a final diagnosis.
IFN-γ Measurement Using IRISA-TB
IFN-γ concentrations were measured in pleural fluid supernatants using the IRISA-TB assay (Antrum Biotech Pty Ltd., Cape Town, South Africa) according to manufacturer instructions. Pleural fluid supernatant was prepared by centrifuging 1 mL of pleural fluid at 3000 g for 15 minutes. The assay was performed in duplicate, and the average values are reported.
Adenosine Deaminase
ADA was performed on the Diazyme assay (Diazyme Laboratories Inc., Poway, CA, USA) using the enzymatic (colorimetric/kinetic) method and in accordance with manufacturer specifications.
Xpert MTB/RIF Ultra
Xpert Ultra assay was performed using 2 mL of pleural fluid diluted with 2 mL of Xpert sample buffer, followed by vigorous mixing. Xpert Ultra cartridges were run on a GeneXpert 4-module system (Dx System, version 4.7b; Cepheid, Sunnyvale, CA, USA) as per manufacturer instructions for a sputum sample.
Statistical Analysis
Diagnostic accuracy, including 95% confidence intervals, were assessed using sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and area under the receiver operator characteristic curve (AUROC) in the definite TPE and non-TPE groups. Unpaired and paired categorical variables were compared using the chi-square and McNemar tests, respectively. Continuous variables were compared using the Student t test, where appropriate. The Mann-Whitney and Wilcoxon rank-sum tests were used for unpaired and paired non-parametric continuous variables, respectively. Statistical analyses were performed using SPSS Statistics (version 28.0; IBM Corp., Armonk, NY, USA) and GraphPad Prism (version 10.1.2; GraphPad Software, Boston, MA, USA).
RESULTS
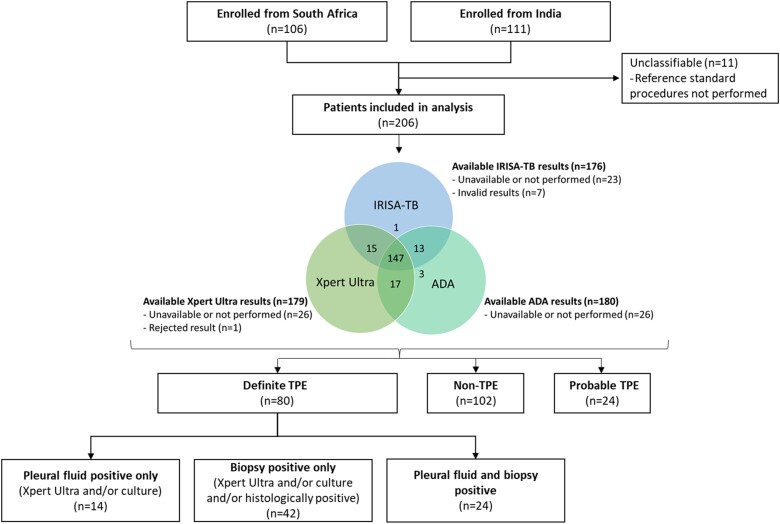
The study overview is illustrated in Figure 1. A total of 217 patients were recruited into the study (106/217 [48.8%] from South Africa and 111/217 [51.2%] from India). After 11 patients were excluded due to having incomplete critical data (unclassifiable), which hindered their correct classification, 206 participants were included in the analysis. Eighty participants had definite TPE, 102 were classified as non-TPE, and 24 participants were classified as probable TPE. A total of 27/102 (26.5%) participants with non-TB effusions had malignancies, including adenocarcinoma, small cell carcinoma, and lymphoma, whereas 27/102 (26.5%) had parapneumonic effusions. There were 27/102 (26.5%) non-TPE participants with an unknown final diagnosis by the time of study completion. This was due to non-specific findings on histology (eg, non-specific inflammatory infiltrate precluding a definitive diagnosis), while 9/102 (8.8%) did not have available histological results. Supplementary Table 1 describes the diagnoses of participants classified as non-TPE.
Figure 1.
Study overview of patient groups. Abbreviations: ADA, adenosine deaminase; TB, tuberculosis; TPE, tuberculous pleural effusion; Xpert Ultra, GeneXpert MTB/RIF Ultra.
Demographic and Clinical Data
Demographic and clinical data are summarized in Table 1. The study population had a median (interquartile range [IQR]) age of 52 (38–65), with individuals with definite TPE being significantly younger compared with the non- and probable TPE groups (42 [31–56] vs 62 [49–70] and 45 [38–66], respectively; P < .001). There were more males than females in our study (135/206 [65.5%] vs 71 [34.5%]). A total of 19/206 (9.2%) individuals had HIV, with a significantly higher proportion in the definite TPE group compared with the non- and probable TPE groups (13/80 [16.3%] vs 5/102 [4.9%] and 1/24 [4.2%], respectively; P = .021). The most reported symptoms across all groups were cough and weight loss. Demographic and clinical characteristics stratified by country are presented in Supplementary Table 2, whereas microbiological and radiological characteristics are described in Supplementary Table 3.
Table 1.
Demographic and Clinical Characteristics
| Total (n = 206) |
Definite TPE (n = 80) | Non-TPE (n = 102) |
Probable TPE (n = 24) |
|
|---|---|---|---|---|
| Age, median (IQR),a y | 52 (38–65) | 42 (31–56) | 62 (49–70) | 45 (38–66) |
| Sex | ||||
| Male | 135 (65.5) | 58 (72.5) | 61 (59.2) | 16 (66.7) |
| Female | 71 (34.5) | 22 (27.5) | 41 (40.2) | 8 (33.3) |
| Current smoker | 42 (20.4) | 14 (17.5) | 25 (24.5) | 3 (12.5) |
| PWHb | 19 (9.2) | 13 (16.3) | 5 (4.9) | 1 (4.2) |
| History of previous TB | 18 (8.7) | 9 (11.3) | 7 (6.9) | 2 (8.3) |
| Symptoms | ||||
| Cough | 130 (63.1) | 53 (66.3) | 59 (57.8) | 18 (75.0) |
| Feverc | 75 (36.4) | 47 (58.8) | 21 (20.6) | 7 (29.2) |
| Weight loss | 143 (69.4) | 55 (68.8) | 68 (66.7) | 20 (83.3) |
| Night sweats | 26 (12.6) | 10 (12.5) | 10 (9.8) | 6 (25.0) |
All numbers are No. (%) unless otherwise indicated.
Abbreviations: IQR, interquartile range; PWH, people with HIV; TB, tuberculosis; TPE, tuberculous pleural effusion; Xpert Ultra, GeneXpert MTB/RIF Ultra.
Superscript letters indicate statistical significance between groups:
a P < .001.
b P = .021.
c P < .001.
Performance Outcomes of IRISA-TB
As shown in Figure 2, the median (IQR) IFN-γ levels were significantly higher in definite TPE than non-TPE (52.3 [22.2–112.4] pg/mL vs 8.8 [4.5–10.4] pg/mL; P < .001). Using the definite and non-TPE groups, at a receiver operating characteristic (ROC) curve–derived rule-in cut-point of 20.5 pg/mL against the composite reference standard, the sensitivity and specificity of IRISA-TB were 81.8% (95% CI, 70.4%–90.2%) and 96.6% (95% CI, 90.3%–99.3%), respectively. The PPV and NPV were 94.7% (95% CI, 85.5%–97.3%) and 87.5% (95% CI, 83.2%–93.0%), respectively. The AUROC for IRISA-TB was 87.6% (95% CI, 80.5%–94.8%) (Figure 3).
Figure 2.
Box plot depicting median (IQR) IFN-γ levels of IRISA-TB (left) and ADA (right) using pleural fluid from patients with definite TPE and non-TPE. Dotted lines represent cut-points (IRISA-TB, 20.5 pg/mL; ADA, 30 IU/mL and 40 IU/mL). Abbreviations: ADA, adenosine deaminase; IQR, interquartile range; interferon gamma, IFN-γ; TB, tuberculosis; TPE, tuberculous pleural effusion.
Figure 3.
Area under the receiver operator characteristic curve for IRISA-TB and ADA. Abbreviations: ADA, adenosine deaminase; TB, tuberculosis; TPE, tuberculous pleural effusion.
Performance Outcomes of ADA
As illustrated in Figure 2, the median (IQR) ADA levels were significantly higher in definite TPE than non-TPE (41.0 [33.0–55.0] IU/mL vs 12.2 [8.1–19.5] IU/mL; P < .001). Against the composite reference standard and using a clinical cut-point of 30 IU/mL (used in South Africa), the sensitivity, specificity, PPV, and NPV of ADA were 83.1% (95% CI, 71.7%–91.2%), 87.1% (95% CI, 78.6%–93.2%), 81.8% (95% CI, 72.4%–88.5%), and 88.0% (95% CI, 81.0%–92.7%), respectively. However, when using a cut-point of 40 IU/mL (as is the case for India), the sensitivity decreased to 53.8% (95% CI, 41.0%–66.3%) while the specificity improved to 92.5% (95% CI, 85.1%–96.9%). The PPV and NPV were 83.3% (95% CI, 70.3%–91.4%) and 74.1% (95% CI, 68.7%–79.0%), respectively. The AUROC for ADA was 92.6 (95% CI, 88.0–97.3) (Figure 3).
Performance Outcomes of Pleural Fluid Xpert MTB/RIF Ultra
Against the composite reference standard, the sensitivity, specificity, PPV, and NPV of Xpert Ultra were 32.9% (95% CI, 22.1%–45.1%), 100.0% (95% CI, 95.9%–100.0%), 100.0% (95% CI, 85.2%–100.0%), and 64.9% (95% CI, 61.1%–68.6%), respectively. The sensitivity of Xpert Ultra was 27.7% (95% CI, 18.3%–39.6%) when Xpert Ultra itself did not form part of the reference standard (ie, culture and/or histopathology only comprised the reference standard).
Comparison of IRISA-TB With Xpert MTB/RIF Ultra and ADA
Table 2 compares the diagnostic accuracy of IRISA-TB with that of other same-day diagnostics (Xpert Ultra and ADA) in the definite TPE group vs the non-TPE group. The sensitivity of IRISA-TB (cut-point 20.5 pg/mL) was significantly better than Xpert Ultra (81.8% [70.4%–90.2%] vs 32.9% [22.1%–45.1%]; P < .001) and ADA at the 40 IU/mL cut-point used in India (81.8% [70.4%–90.2%] vs 53.8% [41.0%–66.3%]; P = .002). The specificity of IRISA-TB was higher than ADA at the 30 IU/mL cut-point used in South Africa (96.6% [90.3%–99.3%] vs 87.1% [78.6%–93.2%]). The PPV of IRISA-TB (94.7% [85.5%–97.3%]) was higher than that of ADA (81.8% [72.4%–88.5%]). The NPV of IRISA-TB was significantly better than that of Xpert Ultra (87.5% [83.2%–93.0%] vs 64.9% [61.1%–68.6%]; P < .001) and ADA (87.5% [83.2%–93.0%] vs 74.1% [68.7%–79.0%]; P < .001) at the 40 IU/mL cut-point. Supplementary Table 4 shows the potential added value of combining tests to improve TPE diagnostic yield.
Table 2.
Diagnostic Accuracy of IRISA-TB, Xpert Ultra, and ADA for the Diagnosis of TPE
| Assaya | TP | FP | TN | FN | Sensitivity (95% CI) |
Specificity (95% CI) |
PPV (95% CI) |
NPV (95% CI) |
Positive LRb (95% CI) |
Negative LR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|
| IRISA-TB (cut-point: 20.5 pg/mL) | ||||||||||
| Total (n = 153) | 54 | 3 | 84 | 12 | 81.8 (70.4–90.2)c,d | 96.6 (90.3–99.3) | 94.7 (85.5–97.3) | 87.5 (83.2–93.0)f,g | 23.7 (7.8–72.6) | 0.2 (0.1–0.3) |
| South Africa (n = 69) | 15 | 1 | 49 | 4 | 78.9 (54.4–94.0) | 98.0 (89.4–99.9) | 93.8 (68.0–99.1) | 92.5 (83.7–96.7) | 39.5 (5.6–278.6) | 0.2 (0.1–0.5) |
| India (n = 84) | 39 | 2 | 35 | 8 | 83.0 (69.2–92.4) | 94.6 (81.8–99.3) | 95.1 (83.4–98.7) | 81.4 (69.9–89.2) | 15.4 (4.0–59.5) | 0.2 (0.1–0.3) |
| Xpert MTB/RIF Ultra | ||||||||||
| Total (n = 157) | 23 | 0 | 87 | 47 | 32.9 (22.1–45.1)c | 100.0 (95.9–100.0) | 100.0 (85.2–100.0) | 64.9 (61.1–68.6)f | … | 0.7 (0.6–0.8) |
| South Africa (n = 68) | 11 | 0 | 47 | 10 | 52.4 (29.8–74.3)e | 100.0 (92.5–100.0) | 100.0 (71.5–100.0) | 82.5 (75.0–88.0)h | … | 0.5 (0.3–0.8) |
| India (n = 89) | 12 | 0 | 40 | 37 | 24.5 (13.3–38.9)e | 100.0 (91.2–100.0) | 100.0 (73.5–100.0) | 51.9 (48.0–55.9)h | … | 0.8 (0.6–0.9) |
| ADA (cut-point: 30 IU/mL) | ||||||||||
| Total (n = 158) | 54 | 12 | 81 | 11 | 83.1 (71.7–91.2) | 87.1 (78.6–93.2) | 81.8 (72.4–88.5) | 88.0 (81.0–92.7) | 6.4 (3.8–11.0) | 0.2 (0.1–0.3) |
| South Africa (n = 78) | 19 | 6 | 50 | 3 | 86.4 (65.1–97.1) | 89.3 (78.1–96.0) | 76.0 (59.4–87.3) | 94.3 (85.3–98.0) | 8.1 (3.7–17.5) | 0.2 (0.1–0.4) |
| India (n = 80) | 35 | 6 | 31 | 8 | 81.4 (66.6–91.6) | 83.8 (68.0–93.8) | 85.4 (73.5–92.5) | 79.5 (67.1–88.0) | 5.0 (2.4–10.6) | 0.2 (0.1–0.4) |
| ADA (cut-point: 40 IU/mL) | ||||||||||
| Total (n = 158) | 35 | 7 | 86 | 30 | 53.8 (41.0–66.3)d | 92.5 (85.1–96.9) | 83.3 (70.3–91.4) | 74.1 (68.7–79.0)g | 7.2 (3.4–15.1) | 0.5 (0.4–0.7) |
| South Africa (n = 78) | 14 | 3 | 53 | 8 | 63.6 (40.7–82.8) | 94.6 (85.1–98.9) | 82.4 (59.8–93.6) | 86.9 (79.2–92.0) | 11.9 (3.8–37.3) | 0.4 (0.2–0.7) |
| India (n = 80) | 21 | 4 | 33 | 22 | 48.8 (33.3–64.5) | 89.2 (74.6–97.0) | 84.0 (66.5–93.3) | 60.0 (52.3–67.2) | 4.5 (1.7–12.0) | 0.6 (0.4–0.8) |
Abbreviations: ADA, adenosine deaminase; FN, false negative; FP, false positive; LR, likelihood ratio; NPV, negative predictive value; PPV, positive predictive value; TB, tuberculosis; TN, true negative; TP, true positive; TPE, tuberculous pleural effusion; Xpert Ultra, GeneXpert MTB/RIF Ultra.
aAvailable results for participants with definite TPE and non-TPE: IRISA-TB (n = 153), Xpert Ultra (n = 157), ADA (n = 158).
bPositive LR for Xpert Ultra indeterminable as specificity is 100.0%.
Superscript letters indicate statistical significance:
c P < .001.
d P = .002.
e P = .023.
f P < .001.
g P < .001.
h P < .001.
Stratifying by country (Table 2), the sensitivity and specificity of IRISA-TB and ADA were generally comparable. However, Xpert Ultra positivity was significantly higher in individuals with definite TPE from South Africa as compared with India (52.4% [29.8%–74.3%] vs 24.5% [13.3%–38.9%]; P = .023).
DISCUSSION
This study evaluated the performance of unstimulated IFN-γ (IRISA–TB) compared with the newer and more sensitive GeneXpert MTB/RIF Ultra for the diagnosis of TPE, where pleural biopsy and pleural fluid microbiological confirmation served as the composite reference standard. The key findings of the study were that (i) IRISA–TB was significantly more sensitive than Xpert Ultra (∼82% vs 33%), with both assays displaying excellent specificity; (ii) IRISA–TB was also significantly more sensitive than ADA at the 40 IU/mL cut-point that is used routinely in India; (iii) IRISA–TB specificity was better than ADA at the 30 IU/mL cut-point used in South Africa (∼97% vs 87%); (iv) the PPV of IRISA–TB was much higher than ADA (∼95% vs 83%) at either the 30 IU/mL or 40 IU/mL cut-point (implying that ∼20% of all positive ADA tests were falsely positive); and (v) the NPV or rule-out value of IRISA–TB (∼88%) was significantly better than that of Xpert Ultra or ADA, which provides the magnitude of probability that TB is unlikely, thus prompting the need for pleural biopsy to confirm an alternative diagnosis.
IRISA–TB showed substantially better sensitivity than Xpert Ultra (∼250% increased sensitivity). TPE is a paucibacillary disease, and although Xpert Ultra has a detection limit of ∼20 organisms/mL [9], the mycobacterial burden in TPE remains below the detection limit of the assay. In such a situation, an immunodiagnostic approach using host biomarkers makes more sense. Such an approach has traditionally used pleural fluid ADA, an enzyme used by mononuclear cells. Indeed, blood-based host immunodiagnostic biomarkers to both predict the development of active TB in the short term (also called incipient TB) [18] and for the diagnosis of active TB [19, 20] show great utility and are being developed as immunodiagnostic blood-based tests. It is therefore not surprising that a host biomarker like unstimulated IFN-γ has a high discriminatory value for the diagnosis of TPE.
TB drives a potent Th1 response, and Th1 cytokines and chemokines are “trapped” within the pleural compartment [21]. The reasons are poorly understood but are related to complex transport mechanisms that govern movement of molecules into and out of compartments. As such, they have excellent diagnostic value as indicated by systematic reviews and meta-analyses [15]. However, while several research-based enzyme-linked immunosorbent assay (ELISA) kits exist for the detection of unstimulated IFN-γ, these run ∼4-hour ELISA protocols, sensitivity is variable, these assays have not been clinically validated, they are prohibitively expensive (∼$500 to $600 per kit), and they do not accommodate single-patient testing (another reason they are cost-prohibitive). Moreover, the antibody pairs required to detect IFN-γ in the serosal compartments are not the same as those that optimally detect IFN-γ in blood given Ph and chemistry considerations, and the heterophile effect (high concentration of molecules and antibodies in serosal compartments, preventing optimal antigen and antibody binding). Antrum Biotech, a University of Cape Town startup, after several years of foundational research, developed a single/multiple-use, low-cost assay (IRISA-TB) that uses a 2-hour protocol and that has been validated for detection of IFN-γ in several compartments [7, 10, 22]. Several studies have shown IRISA–TB to be highly sensitive and specific for the diagnosis of pleural TB, with considerably higher sensitivity and specificity than Xpert in rigorous studies using pleural biopsy and fluid microbiology as the reference standard [7, 10]. The same was shown for pericardial TB [22].
IRISA–TB also previously showed improved performance compared with ADA (the default assay used in many TB-endemic settings as pleural biopsy is often not accessible). In the South African setting, where the 30 IU/mL cut-point is used, the specificity of IRISA–TB was better than ADA, which results in significant misclassification bias such that ∼1 in 7 to 8 persons would be erroneously diagnosed with TB when they did not have TPE. This results in huge additional cost to the health care system and avoidable anxiety and adverse events to individuals, which may be fatal (not to mention absence from schoolwork and the anxiety due to social stigmatization). In the South African setting, where there are ∼80 000 newly diagnosed patients with EPTB annually (and assuming that ∼40 000 have pleural TB and hence ∼400 000 patients would need to be screened with ADA at a disease prevalence of 10% in those with presumed TPE) [23], given the lower specificity of ADA, we estimate that this would conservatively result in ∼53 000 false treatment starts for TPE. Indeed, a recently published cost-effectiveness study on IRISA–TB in South Africa showed that the assay was highly cost-effective as it prevented false-positive treatment starts due to its high specificity [23]. The cost savings amounted to ∼US$45 million per annum in the South African setting. In the Indian setting, where an ADA cut-point of 40 IU/mL is used (resulting in higher specificity, thus avoiding false treatment starts), the sensitivity is only ∼50% to 60%, thus missing a large proportion of individuals with TPE. The NPV is also important in the context of a presumed TPE as it provides the probability of ruling out TB, thus prompting the search for alternative diagnoses through a pleural biopsy. IRISA–TB NPV was considerably higher than that of Xpert Ultra, thus providing clinical value as it is helpful to guide further investigation. The PPV of ADA at either cut-point (30 IU/mL or 40 IU/mL) was only ∼80% (ie, of all positive tests, 20% don’t have TB), while for IRISA–TB and Xpert it was ∼95%–100%. Given these considerations, it is not surprising that IRISA–TB was more cost-effective than ADA [23].
It has been proposed that two cut-points of ADA be used with the lower cut-point to rule out TB and a higher cut-point to rule in TB [24]. However, besides being extremely confusing for junior clinicians, such an approach would lead to decision paralysis in a significant number of persons whose ADA results would fall between the two cut-points (an indeterminate result rendering the approach itself impractical). Furthermore, it has been proposed that ADA should be combined with pleural fluid cytology (lymphocyte count) as it may increase the specificity of ADA for the diagnosis of pleural TB [24–26]. However, the standard of care in most TB-endemic countries (especially those in Africa) is not to perform pleural fluid cytology. Indeed, it is not something that is routinely done in our setting, and hence we did not have the lymphocyte percentages available for all individuals. Moreover, cytologists and histopathologists are in short supply in endemic countries, which also adds to the overall cost of diagnosis.
IRISA–TB uses unprocessed pleural fluid (unfiltered, unstimulated, and not antigen challenged), unlike IGRA, which uses highly processed material and overnight stimulation, and the biological material undergoes challenge with TB-specific antigens. We have previously evaluated IGRA for the diagnosis of pleural TB [16]. Although it has some value, a substantial proportion of assays are indeterminate, limiting its utility. Furthermore, the cost and additional incubation/stimulation step make this assay redundant because the same (antigen stimulation of Th1 lymphocytes) has already happened in vivo within the pleural compartment where IFN-γ is effectively trapped and is present in high concentrations. Thus, further processing of the sample is unnecessary. We had previously evaluated other Th1 biomarkers like IP10 and pleural fluid, but sensitivity was lower than that of IFN-γ [6].
There are several strengths to our study. First, pleural biopsy and pleural fluid microbiology were undertaken in all individuals, thus substantially reducing the misclassification bias that other studies were prone to. Most studies use only microbiologically positive fluid samples (culture positive), which overestimates polymerase chain reaction test performance, and it further excludes the majority of paucibacillary samples. Second, we evaluated ADA at both commonly used cut-points (30 IU/mL being the high-sensitivity cut-point and 40 IU/mL the high-specificity cut-point). Third, we compared results across different settings and in different subpopulations including people with HIV (PWH). However, our study also had several limitations. It would have been beneficial to have a larger sample size and thus tighter confidence intervals to our estimates. However, this is one of the largest published studies on pleural fluid TB diagnostics. Our study was unable to obtain good estimates of performance of IRISA–TB in PWH. However, previous studies of IRISA–TB showed excellent performance in the PWH subgroup and better performance than in HIV-negative persons [7, 10]. Approximately one-third of non-TPE participants did not have a final diagnosis by the time of the study completion; however, all available results were negative for TPE as per study definitions. The IRISA–TB assay is laboratory-based and not point-of-care. Nevertheless, a simple ELISA plate reader is found in almost all basic laboratories in TB-endemic countries, and a point-of-care version (in lateral flow format) is already in the advanced stages of development and testing. We were unable to include differential cell counts (lymphocyte count) in the analysis, which may have impacted ADA accuracy estimates. Finally, the sample size was limited (80 persons with definite TB using histopathology and microbiology as the reference), and the impact on treatment outcomes using this technology has not been ascertained. However, this ranks among one of the larger studies incorporating pleural biopsy as a reference (error margins varied by ∼10% for IRISA-TB), and a large multicentric European Union–funded study across four African countries is currently underway to address this limitation (EDCTP ref. RIA101103281) [27].
In conclusion, IRISA–TB has a high sensitivity and NPV (rule-out value) for the diagnosis of pleural TB and is thus superior in performance to both Xpert Ultra and ADA.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Acknowledgments
We are indebted to the participants who took part in this study. We thank the Health Directorate of the City of Cape Town for providing access to appropriate health care facilities. We further acknowledge the assistance of the University of Cape Town Research Ethics Committee, the clinical staff at each of the TB clinics, community leaders, and the University of Cape Town Lung Institute TB community advisory board for enabling this study.
Data availability. Deidentified data from this study are available upon reasonable request to the corresponding author.
Patient consent and ethical approval. Written informed consent was obtained from all participants. This study was ethically approved by the University of Cape Town Human Research Ethics Committee (HREC ref. 526/2018, September 17, 2018) and the Christian Medical College Office of Research Institutional Review Board (IRB: 10377 [DIAGNO], June 27, 2018).
Financial support . This study was partly funded by the Department of Biotechnology (DBT), India, and the National Institute of Allergy and Infectious Diseases (NIAID), USA. The study was administered by the Civilian Research and Development Foundation (CRDF Global) (DAA3-18-64075) under the aegis of the RePORT India and South Africa Consortium. This work was partially funded by the South African Medical Research Council (grant no. RFA-EMU-02-2017). K.D.'s lab also acknowledges funding from the European and Developing Countries Clinical Trials Partnership (EDCTP: grant nos. TMA-2015SF-1043 and TMA-1051-TESAII), UK Medical Research Council (grant no. MR/S03563X/1), and the Wellcome Trust (grant no. MR/S027777/1). A.E. acknowledges funding from the EDCTP (grant no. TMA-2015CDF-1052). The funders did not influence the results of this research in any way. The IRISA-TB kits were donated by Antrum Biotech. However, Antrum Biotech and its associates had no role in the study design, recruitment of patients, or analysis of the data and were blinded to the patients’ clinical diagnoses and classification of patient subgroups. P.R. is an employee of Antrum Biotech, the company that developed the IRISA-TB test. She provided training to laboratory technicians who performed the assay for this project and assisted with the performance of quality checks on the kits.
Potential conflicts of interest . All authors: No reported conflicts.
Contributor Information
Devasahayam J Christopher, Department of Pulmonary Medicine, Christian Medical College, Vellore, India.
Aliasgar Esmail, Centre for Lung Infection and Immunity, Division of Pulmonology, Department of Medicine and University of Cape Town Lung Institute, Cape Town, South Africa; South African MRC/UCT Centre for the Study of Antimicrobial Resistance, University of Cape Town, Cape Town, South Africa.
Alex J Scott, Centre for Lung Infection and Immunity, Division of Pulmonology, Department of Medicine and University of Cape Town Lung Institute, Cape Town, South Africa; South African MRC/UCT Centre for the Study of Antimicrobial Resistance, University of Cape Town, Cape Town, South Africa.
Lindsay Wilson, Centre for Lung Infection and Immunity, Division of Pulmonology, Department of Medicine and University of Cape Town Lung Institute, Cape Town, South Africa; South African MRC/UCT Centre for the Study of Antimicrobial Resistance, University of Cape Town, Cape Town, South Africa.
Philippa Randall, Centre for Lung Infection and Immunity, Division of Pulmonology, Department of Medicine and University of Cape Town Lung Institute, Cape Town, South Africa.
Balamugesh Thangakunam, Department of Pulmonary Medicine, Christian Medical College, Vellore, India.
Deepa Shankar, Department of Pulmonary Medicine, Christian Medical College, Vellore, India.
Sekar Rajasekar, Department of Pulmonary Medicine, Christian Medical College, Vellore, India.
Christhunesa S Christudass, Division of Neurochemistry, Department of Neurological Sciences, Christian Medical College, Vellore, India.
Louié Kühn, Centre for Lung Infection and Immunity, Division of Pulmonology, Department of Medicine and University of Cape Town Lung Institute, Cape Town, South Africa; South African MRC/UCT Centre for the Study of Antimicrobial Resistance, University of Cape Town, Cape Town, South Africa.
Jeremi Swanepoel, Centre for Lung Infection and Immunity, Division of Pulmonology, Department of Medicine and University of Cape Town Lung Institute, Cape Town, South Africa; South African MRC/UCT Centre for the Study of Antimicrobial Resistance, University of Cape Town, Cape Town, South Africa.
Tahlia Perumal, Centre for Lung Infection and Immunity, Division of Pulmonology, Department of Medicine and University of Cape Town Lung Institute, Cape Town, South Africa; South African MRC/UCT Centre for the Study of Antimicrobial Resistance, University of Cape Town, Cape Town, South Africa.
Anil Pooran, Centre for Lung Infection and Immunity, Division of Pulmonology, Department of Medicine and University of Cape Town Lung Institute, Cape Town, South Africa; South African MRC/UCT Centre for the Study of Antimicrobial Resistance, University of Cape Town, Cape Town, South Africa.
Suzette Oelofse, Centre for Lung Infection and Immunity, Division of Pulmonology, Department of Medicine and University of Cape Town Lung Institute, Cape Town, South Africa; South African MRC/UCT Centre for the Study of Antimicrobial Resistance, University of Cape Town, Cape Town, South Africa.
Keertan Dheda, Centre for Lung Infection and Immunity, Division of Pulmonology, Department of Medicine and University of Cape Town Lung Institute, Cape Town, South Africa; South African MRC/UCT Centre for the Study of Antimicrobial Resistance, University of Cape Town, Cape Town, South Africa; Department of Immunology and Infection, Faculty of Infectious and Tropical Diseases, London School of Hygiene and Tropical Medicine, London, UK.
References
- 1. World Health Organization. Global Tuberculosis Report 2023. World Health Organization ; 2023. . Licence: CC BY-NC-SA 3.0 IGO.
- 2. World Health Organization. WHO COVID-19 dashboard . 2020. Available at: https://covid19.who.int/. Accessed January 1, 2024.
- 3. Karstaedt AS. Extrapulmonary tuberculosis among adults: experience at Chris Hani Baragwanath Academic Hospital, Johannesburg, South Africa. S Afr Med J 2013; 104:22–4. [DOI] [PubMed] [Google Scholar]
- 4. Antonangelo L, Faria CS, Sales RK. Tuberculous pleural effusion: diagnosis & management. Expert Rev Respir Med 2019; 13:747–59. [DOI] [PubMed] [Google Scholar]
- 5. Pandie S, Peter JG, Kerbelker ZS, et al. The diagnostic accuracy of pericardial and urinary lipoarabinomannan (LAM) assays in patients with suspected tuberculous pericarditis. Sci Rep 2016; 6:32924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dheda K, Van-Zyl Smit RN, Sechi LA, et al. Clinical diagnostic utility of IP-10 and LAM antigen levels for the diagnosis of tuberculous pleural effusions in a high burden setting. PLoS One 2009; 4:e4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Meldau R, Peter J, Theron G, et al. Comparison of same day diagnostic tools including GeneXpert and unstimulated IFN-γ for the evaluation of pleural tuberculosis: a prospective cohort study. BMC Pulm Med 2014; 14:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dorman SE, Schumacher SG, Alland D, et al. Xpert MTB/RIF ultra for detection of Mycobacterium tuberculosis and rifampicin resistance: a prospective multicentre diagnostic accuracy study. Lancet Infect Dis 2018; 18:76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Esmail A, Tomasicchio M, Meldau R, Makambwa E, Dheda K. Comparison of Xpert MTB/RIF (G4) and Xpert Ultra, including trace readouts, for the diagnosis of pulmonary tuberculosis in a TB and HIV endemic setting. Int J Infect Dis 2020; 95:246–52. [DOI] [PubMed] [Google Scholar]
- 10. Meldau R, Randall P, Pooran A, et al. Same-day tools, including Xpert Ultra and IRISA-TB, for rapid diagnosis of pleural tuberculosis: a prospective observational study. J Clin Microbiol 2019; 57:e00614–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aggarwal AN, Agarwal R, Sehgal IS, Dhooria S. Adenosine deaminase for diagnosis of tuberculous pleural effusion: a systematic review and meta-analysis. PLoS One 2019; 14:e0213728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Patel VB, Singh R, Connolly C, Kasprowicz V, Ndung'u T, Dheda K. Comparative utility of cytokine levels and quantitative RD-1-specific T cell responses for rapid immunodiagnosis of tuberculous meningitis. J Clin Microbiol 2011; 49:3971–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Greco S, Girardi E, Masciangelo RA, Capoccetta GB, Saltini C. Adenosine deaminase and interferon gamma measurements for the diagnosis of tuberculous pleurisy: a meta-analysis. Int J Tuberc Lung Dis 2003; 7:777–86. [PubMed] [Google Scholar]
- 14. Santos AP, Corrêa RDS, Ribeiro-Alves M, et al. Application of Venn's diagram in the diagnosis of pleural tuberculosis using IFN-γ, IP-10 and adenosine deaminase. PLoS One 2018; 13:e0202481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aggarwal AN, Agarwal R, Dhooria S, Prasad KT, Sehgal IS, Muthu V. Unstimulated pleural fluid interferon gamma for diagnosis of tuberculous pleural effusion: a systematic review and meta-analysis. J Clin Microbiol 2021; 59:e02112–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dheda K, van Zyl-Smit RN, Sechi LA, et al. Utility of quantitative T-cell responses versus unstimulated interferon-γ for the diagnosis of pleural tuberculosis. Eur Respir J 2009; 34:1118–26. [DOI] [PubMed] [Google Scholar]
- 17. TB DOTS Strategy Coordination . National Tuberculosis Management Guidelines. National Department of Health; 2014. [Google Scholar]
- 18. Zak DE, Penn-Nicholson A, Scriba TJ, et al. A blood RNA signature for tuberculosis disease risk: a prospective cohort study. Lancet 2016; 387:2312–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rakotosamimanana N, Richard V, Raharimanga V, et al. Biomarkers for risk of developing active tuberculosis in contacts of TB patients: a prospective cohort study. Eur Respir J 2015; 46:1095–103. [DOI] [PubMed] [Google Scholar]
- 20. Berry MP, Graham CM, McNab FW, et al. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature 2010; 466:973–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. da Cunha Lisboa V, Ribeiro-Alves M, da Silva Corrêa R, et al. Predominance of th1 immune response in pleural effusion of patients with tuberculosis among other exudative etiologies. J Clin Microbiol 2019; 58:e00927–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pandie S, Peter JG, Kerbelker ZS, et al. Diagnostic accuracy of quantitative PCR (Xpert MTB/RIF) for tuberculous pericarditis compared to adenosine deaminase and unstimulated interferon-γ in a high burden setting: a prospective study. BMC Med 2014; 12:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pooran A, Randall P, Meldau R, Maine R, Esmail A. Economic evaluation of microbiological and host biomarker-based tests for the diagnosis of pleural tuberculosis in a high burden setting. J Thorac Dis 2022; 14:3167–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shaw JA, Ahmed L, Koegelenberg CFN. Effusions related to TB. Eur Respir Monogr 2020; 87:172–92. [Google Scholar]
- 25. Burgess LJ, Maritz FJ, Le Roux I, Taljaard JF. Combined use of pleural adenosine deaminase with lymphocyte/neutrophil ratio: increased specificity for the diagnosis of tuberculous pleuritis. Chest 1996; 109:414–9. [DOI] [PubMed] [Google Scholar]
- 26. Garcia-Zamalloa A, Taboada-Gomez J. Diagnostic accuracy of adenosine deaminase and lymphocyte proportion in pleural fluid for tuberculous pleurisy in different prevalence scenarios. PLoS One 2012; 7:e38729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.National Library of Medicine. Same day diagnosis of extrapulmonary TB (TB serositis and TB meningitis: Epi-TB). NCT06135818. Available at: https://clinicaltrials.gov/study/NCT06135818. Accessed January 11, 2024.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.