Abstract
Triglyceride-glucose (TyG) index has emerged as a novel biomarker for detecting insulin resistance (IR) and has been proven to be associated with various diseases. However, its correlation with the prognosis of severe sepsis remains unraveled. This retrospective cohort study utilized patient records from the Medical Information Mart for Intensive Care (MIMIC-IV, version 2.2) to examine the outcomes of patients with sepsis. The primary outcomes were hospital mortality and intensive care unit (ICU) mortality. The correlation between the TyG index and outcomes was evaluated through the Kaplan-Meier method, the Log-rank test, and univariate and multivariate Cox regression analyses. Additionally, restricted cubic spline (RCS) regression analysis was employed to delve into the nonlinear relationship between baseline TyG index and outcomes, with trend significance assessed through quartile levels. Subgroup analyses were conducted to evaluate the consistency of the TyG index’s prognostic value across various influencing factors. The study included 1,742 patients with sepsis requiring intensive care. The in-hospital mortality rate was 19.75% (344/1,742), and the ICU mortality rate was 14.75% (257/1,742). Cox regression analysis revealed that, in comparison to the first quartile (Q1), patients in the fourth quartile (Q4) had a 63% higher risk of in-hospital mortality (HR 1.63 [95% CI 1.22 to 2.18], P < 0.01) and a 79% higher risk of ICU mortality (HR 1.79 [95% CI 1.28 to 2.51], P < 0.001). Model 3 showed that ICU mortality risks for Q4, Q3, and Q2 were 240%, 75%, and 33% higher, respectively (HR 3.40 [95% CI 2.24 to 5.16], P < 0.001; HR 1.75 [95% CI 1.16 to 2.63], P = 0.007; HR 1.33 [95% CI 1.20 to 1.53], P < 0.001). RCS regression analysis identified a nonlinear association between the TyG index and mortality (overall P < 0.001; P for nonlinearity < 0.001, with an inflection point at 8.9). Subgroup analysis showed that the effect size and direction were consistent across different subgroups, suggesting the stability of the results. This study demonstrates that a higher TyG index is significantly associated with increased in-hospital and ICU mortality risk in critically ill sepsis patients, with evidence of non-linear correlation. Therefore, the TyG index helps identify the mortality prognosis of sepsis patients in the ICU.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-75050-8.
Keywords: Intensive care unit, MIMIC, Mortality, Sepsis, Triglyceride glucose index
Subject terms: Biomarkers, Diseases, Endocrinology, Medical research, Pathogenesis, Risk factors
Background
Sepsis is a life-threatening organ dysfunction caused by an imbalance in the body’s response to infection. The incidence of blood glucose metabolism disorder is relatively high in sepsis patients, and its pathophysiology is that when the body is infected, a large amount of pro-inflammatory mediators is released, leading to peripheral insulin resistance1. This is typically manifested as hyperglycemia and increased glycemic variability (GV)2. Despite plenty of studies3–5 elucidating the mechanisms of glucose metabolism disorders, the interplay between overall blood glucose and GV remains unclear. Early studies6,7 demonstrated that infection and inflammation can lead to significant changes in lipids and lipoproteins, resulting in a decrease in lipid and lipoprotein levels in sepsis patients. Lower baseline lipid levels are correlated with an increased risk of sepsis and poorer prognosis8. It has been proved that9 decreased plasma triglyceride (TG) levels are linked to higher mortality in sepsis patients, though its use as a prognostic marker remains debated. At present, the pathological and physiological mechanisms by which blood lipids affect the prognosis of sepsis patients are not well understood, and whether various lipoproteins can serve as predictive biomarkers and therapeutic targets for sepsis and renal dysfunction has not been investigated.
With the extensive research on insulin sensitivity parameters, several simple insulin sensitivity parameters that can be applied clinically and accurately reflect patients’ insulin sensitivity have gradually been validated, and the triglyceride-glucose (TyG) index is one of them10. The TyG index is positively correlated with contrast-induced nephropathy, risk of ischemic stroke11, and severity and prognosis of coronary artery disease12, which is supported by moderate-quality evidence. Although prior studies13,14 have highlighted the potential prognostic value of TyG in sepsis patients, they have not investigated the survival time of the population or the presence of a nonlinear association between the TyG index and short-term mortality risk in sepsis patients. Therefore, the relationship between the TyG index and mortality rate in sepsis patients remains unraveled.
In the Surviving Sepsis Campaign (SSC), early identification and improved prognosis were two major goals of sepsis treatment15, and the application of improvement plans for sepsis patients, including sepsis screening, intervention effectiveness assessment, and patient prognosis evaluation are recommended16. Therefore, it is urgent to identify novel biomarkers that can predict the prognosis of sepsis. This study employed the open-source critical care database, multiple statistical models, survival analysis, and subgroup analysis to shed light on the association between the TyG index and hospital/ICU mortality rate in sepsis patients.
Methods
Data source and study design
We conducted a retrospective cohort study based on data from MIMIC IV (version 2.2), which included two in-house database systems: a custom hospital-wide electronic health record (EHR) and an ICU-specific clinical information system from 2008 to 202417. Access to the database was obtained by one of the authors (JQ L), who obtained the necessary authentication and completed the Collaborative Institutional Training Initiative examination (authentication number 60691748). Relevant variables for our study were extracted, and de-identification of patients was performed to ensure their privacy. Due to the retrospective nature of the study, given the anonymity of patient health information in this database, the need of informed consent was waived by the Human Research Ethics Committee of Ningbo No.2 Hospital.
Due to the retrospective nature of our study, traditional sample size calculations or power analyses were not conducted. Nevertheless, the number of available records in the database was considered sufficient to fulfill the objectives. This assessment is grounded in the aim to investigate the relationship between the TyG index and mortality outcomes within a large and diverse patient population. The convenience samples drawn from the database effectively represent the intensive care population, thereby supporting the statistical analyses employed in this study.
Participants
The study included all sepsis patients from the MIMIC IV v2.2 database. Sepsis was defined according to the Sepsis 3.0 criteria jointly established by the American Society for Critical Care Medicine (SCCM) and the European Society for Critical Care Medicine (ESICM)18,19. Patient data were extracted with the help of PostgreSQL, with inclusion criteria20–22 being sepsis patients aged over 18 and admitted to the ICU for the first time. Exclusion criteria were: (1) Patients were under 18 years old; (2) The ICU stay is less than 48 h; (3) There were multiple ICU admissions due to sepsis; (4) Data were insufficient. For instance, the is a lack of records for triglycerides and fasting blood glucose.
Research procedures and definitions
Data extraction from MIMIC-IV was conducted with the help of Structured Query Language (SQL) through Navicat Premium. The extracted data included patient demographics (age, height, weight, gender, insurance, race, marital status), medical history (hypertension, type 2 diabetes, heart failure, myocardial infarction, malignant tumors, chronic renal failure, cirrhosis, hepatitis, tuberculosis, pneumonia, chronic obstructive pulmonary disease, hyperlipidemia), and initial laboratory test results (white blood cell count, red blood cell count, neutrophil count, lymphocyte count, platelet count, hemoglobin count, mean corpuscular volume, hematocrit, albumin, globulin, total protein, sodium, potassium, calcium, chloride, blood glucose, glycosylated hemoglobin (HbA1c), anion gap, blood pH, arterial partial pressure of carbon dioxide, arterial partial pressure of oxygen, lactate, total carbon dioxide, calcium fluoride, thrombin time, fibrinogen, partial thromboplastin time, international normalized ratio, D-D dimer, triglycerides, total cholesterol, high-density lipoprotein, low-density lipoprotein, total bilirubin, direct bilirubin, indirect bilirubin, aspartate aminotransferase, alanine aminotransferase, urea nitrogen, creatinine, serum lactate dehydrogenase, creatine kinase, creatine kinase isoenzyme, troponin, N-terminal B-type natriuretic peptide precursor, urinary sugar, urinary albumin), special treatments (continuous renal replacement therapy and mechanical ventilation), clinical scores (SOFA score, APACHE III score, SAPS II score, Oasis score, Charlson score, SIRS score, and GCS score), and clinical outcomes (length of hospital stay, in-hospital mortality, ICU stay, and ICU mortality). Predictors with more than 30% missing data were excluded during data cleaning. The TyG index was calculated through the formula: TyG index = ln [triglycerides (mg/dL) × glucose (mg/dL)/2]23.
Outcomes and measures
The primary outcomes of this study were hospital mortality and ICU mortality.
Statistical analysis
Continuous variables were presented as mean ± standard deviation or median (interquartile range), while categorical variables were reported as frequency and percentage. Data conforming to a normal distribution were analyzed through the t-test or analysis of variance (ANOVA). For data not following a normal distribution, the Mann-Whitney U test or Kruskal-Wallis test was employed. Kaplan-Meier survival analysis was utilized to assess the incidence of endpoint events across different TyG index levels, with differences evaluated through the log-rank test. Kaplan-Meier curves offer a visual comparison of survival differences between groups or conditions and do not require prior assumptions about data distribution, so it was relatively flexible in use. The Cox proportional hazards model was utilized to calculate the hazard ratio (HR) and 95% confidence interval (CI) between the TyG index and the endpoint. This model, taking survival outcome and survival time as dependent variables, enabled simultaneous analysis of multiple factors affecting survival and analysis of the data with censored survival time, and did not necessitate the estimation of the survival distribution type. The TyG index was analyzed both as a continuous variable and by quartiles. Models were adjusted as follows: Model 1 involved univariate analysis; Model 2 included adjustment for age, gender, height, weight, and race based on Model 1; and Model 3 was further adjusted for insurance status, marble status, white blood cell count (WBC), red blood cell count (RBC), hemoglobin, red cell distribution width (RDW), albumin, chloride, alanine aminotransferase (ALT), aspartate aminotransferase (AST), SOFA score, APS III score, SAPS II score, OASIS score, Charlson comorbidity index, Glasgow Coma Scale (GCS), hypertension (HT), type 2 diabetes (DM2), heart failure (HF), myocardial infarction (MI), myocardial trauma (MT), chronic kidney disease (CKD), acute renal failure (ARF), stroke, hyperlipidemia (HLP), as well as chronic obstructive pulmonary disease (COPD). Additionally, a restricted cubic spline (RCS) regression model was applied to examine the nonlinear relationship between baseline TyG index and mortality in both hospital and ICU settings. The TyG index was inputted as either a continuous or ordered variable into the model, with the first quartile of the TyG index serving as the reference group. The quartile level was used for the calculation of the P-value of the trend. RCS was a non-parametric flexible fitting method that models survival curves by transforming survival times into piecewise functions at individual nodes and can accommodate various types of survival time distributions without excessive assumptions. Subgroup analyses were conducted to explore potential differences across various subgroups based on age (≤ 70 and > 70), sex, BMI (< 27.4 kg/m², 27.4–31.2 kg/m², ≥ 31.2 kg/m²), hypertension, type 2 diabetes, heart failure, continuous renal replacement therapy, and ventilation, to evaluate the consistency of the TyG index’s prognostic value for the primary outcomes. Cox models were also adopted in subgroup analyses to adjust for all variables in the patient’s baseline information. Data processing and analysis were carried out via R version 4.3.0, with statistical significance set at P < 0.05 for two-tailed tests. For missing values in the data, the multiple imputation method of the random forest was used to interpolate the missing value data (through the R package “mice”). Features with missing values exceeding 50% were removed before interpolation.
Results
Among the adult patients in the MIMIC-IV database, a total of 22,518 subjects met the eligibility criteria. From the database, 135 prognostic factors were initially extracted. Following data cleaning, 50 predictors with over 30% missing data were excluded. In the end, 85 forecast factors were included in the model.
Characteristics of included patients
A total of 1,742 patients with sepsis who met the study’s inclusion criteria were included. The screening process is depicted in Fig. 1. The mean age of the patients was 63.9 ± 17.2 years. The male-to-female ratio was 1.338:1 (997 males to 745 females). The in-hospital mortality rate was 19.75% (344/1,742), the ICU mortality rate was 14.75% (257/1,742), and the overall mortality rate was 42.54% (741/1,742). The median length of hospital stay was 12.61 days for survivors and 10.07 days for non-survivors. The 1,742 patients were categorized into Q1 (Quartile 1; TyG index ≤ 8.56, n = 436), Q2 (Quartile 2; 8.56 < TyG index ≤ 9.03, n = 435), Q3 (Quartile 3; 9.03 < TyG index ≤ 9.56, n = 435), and Q4 (Quartile 4; TyG index > 9.56, n = 436). The median (interquartile range) of the TyG index was 9.03 (8.56–9.56). Upon stratification into these four categories, the distribution of each variable across the groups was analyzed. All baseline data are presented in Table 1 and Supplementary Table 1. Notably, patients in the Q1 group were significantly older compared to those in the Q4 group. Conversely, patients in the Q1 group were notably smaller in weight and height, and exhibited a higher proportion of females THAN the Q2 group, which had a higher proportion of males. From Q1 to Q4, there was an observed upward trend in WBC, neutrophil, glucose, HbA1c, aniongap, fibrinogen, TG, TC, BUN, creatinine, and BLD, while albumin, total calcium, HDL-C showed a downward trend. Additionally, the Q4 group had a longer duration of mechanical ventilation in comparison to the Q1 group. Regarding past medical history, we found a sequential increase in the number of patients diagnosed with type 2 diabetes, ARF, and AKI from Q1 to Q4, whereas the number of patients with previous stroke decreased sequentially.
Fig. 1.
Selection of the study population from the MIMIC-IV database.
Table 1.
Crucial characteristics and outcomes of participants categorized by the TyG index.
| Variables | Total (n = 1,742) | Q1 (n = 436) | Q2 (n = 435) | Q3 (n = 435) | Q4 (n = 436) | F/χ² | P |
|---|---|---|---|---|---|---|---|
| Characteristics | |||||||
| Age (years) | 63.90 ± 17.21 | 67.71 ± 18.54 | 65.72 ± 16.19 | 63.30 ± 16.91 | 58.88 ± 15.87 | 22.05 | < 0.001 |
| Weight (kg) | 86.23 ± 26.44 | 78.01 ± 22.93 | 83.87 ± 26.41 | 87.28 ± 26.18 | 95.62 ± 26.98 | 35.44 | < 0.001 |
| Height (cm) | 169.90 ± 10.86 | 169.00 ± 10.06 | 169.85 ± 11.08 | 169.34 ± 11.54 | 171.10 ± 10.55 | 2.19 | 0.087 |
| Gender (n(%)) | 6.66 | 0.084 | |||||
| F | 745 (42.77) | 204 (46.79) | 193 (44.37) | 179 (41.15) | 169 (38.76) | ||
| M | 997 (57.23) | 232 (53.21) | 242 (55.63) | 256 (58.85) | 267 (61.24) | ||
| Race (n(%)) | 14.33 | 0.280 | |||||
| Asian | 42 (2.41) | 11 (2.52) | 10 (2.30) | 15 (3.45) | 6 (1.38) | ||
| Black | 146 (8.38) | 46 (10.55) | 36 (8.28) | 35 (8.05) | 29 (6.65) | ||
| Hispanic | 48 (2.76) | 12 (2.75) | 9 (2.07) | 15 (3.45) | 12 (2.75) | ||
| Other/Unknown | 478 (27.44) | 105 (24.08) | 117 (26.90) | 119 (27.36) | 137 (31.42) | ||
| White | 1028 (59.01) | 262 (60.09) | 263 (60.46) | 251 (57.70) | 252 (57.80) | ||
| Insurance (n(%)) | 12.08 | 0.060 | |||||
| Medicaid | 142 (8.15) | 35 (8.03) | 34 (7.82) | 37 (8.51) | 36 (8.26) | ||
| Medicare | 734 (42.14) | 207 (47.48) | 186 (42.76) | 183 (42.07) | 158 (36.24) | ||
| Other | 866 (49.71) | 194 (44.50) | 215 (49.43) | 215 (49.43) | 242 (55.50) | ||
| Marital Status (n(%)) | 21.49 | 0.011 | |||||
| Divorced | 120 (8.33) | 25 (6.70) | 40 (11.43) | 30 (8.17) | 25 (7.12) | ||
| Married | 670 (46.50) | 168 (45.04) | 152 (43.43) | 176 (47.96) | 174 (49.57) | ||
| Single | 471 (32.69) | 118 (31.64) | 117 (33.43) | 111 (30.25) | 125 (35.61) | ||
| Widowed | 180 (12.49) | 62 (16.62) | 41 (11.71) | 50 (13.62) | 27 (7.69) | ||
| Laboratory parameters | |||||||
| WBC (×109/L) | 13.48 ± 10.78 | 11.66 ± 8.40 | 12.93 ± 11.26 | 14.55 ± 9.60 | 14.76 ± 13.02 | 8.06 | < 0.001 |
| RBC (×1012/L) | 3.67 ± 0.75 | 3.66 ± 0.72 | 3.71 ± 0.78 | 3.67 ± 0.75 | 3.63 ± 0.74 | 0.93 | 0.427 |
| Neutrophil (×109/L) | 12.04 ± 9.07 | 9.31 ± 6.10 | 11.10 ± 6.49 | 14.18 ± 12.95 | 12.77 ± 7.86 | 7.07 | < 0.001 |
| Lymphocytes (×109/L) | 2.04 ± 12.81 | 1.12 ± 1.43 | 3.03 ± 19.28 | 1.45 ± 2.74 | 2.50 ± 16.25 | 0.59 | 0.622 |
| Platelet (×109/L) | 202.89 ± 112.56 | 196.85 ± 104.22 | 211.09 ± 119.22 | 211.09 ± 117.61 | 192.55 ± 107.62 | 3.19 | 0.023 |
| RDW (%) | 15.20 ± 2.40 | 15.05 ± 2.45 | 15.21 ± 2.49 | 15.24 ± 2.36 | 15.31 ± 2.29 | 0.89 | 0.444 |
| Albumin (g/L) | 3.08 ± 0.66 | 3.24 ± 0.62 | 3.13 ± 0.67 | 3.10 ± 0.66 | 2.90 ± 0.65 | 13.42 | < 0.001 |
| Chlorine (mmol/L) | 104.27 ± 6.27 | 104.43 ± 5.89 | 104.79 ± 5.87 | 104.50 ± 6.08 | 103.37 ± 7.09 | 4.34 | 0.005 |
| Glucose (mmol/L) | 151.31 ± 61.38 | 116.06 ± 29.35 | 134.78 ± 36.23 | 158.40 ± 50.80 | 196.00 ± 82.23 | 179.77 | < 0.001 |
| HbA1c (mmol/L) | 6.24 ± 1.59 | 5.74 ± 0.99 | 5.91 ± 0.89 | 6.23 ± 1.30 | 7.52 ± 2.56 | 42.59 | < 0.001 |
| TG (mg/dL) | 186.48 ± 323.39 | 68.50 ± 20.81 | 106.24 ± 28.30 | 148.06 ± 47.29 | 422.84 ± 580.72 | 132.40 | < 0.001 |
| TC (mg/dL) | 147.19 ± 56.06 | 135.48 ± 44.63 | 147.07 ± 51.75 | 143.46 ± 52.52 | 171.25 ± 74.06 | 15.85 | < 0.001 |
| Treatment | |||||||
| CRRT (days) | 6.00 ± 5.71 | 5.17 ± 3.20 | 4.89 ± 4.78 | 7.49 ± 7.51 | 5.95 ± 5.48 | 1.67 | 0.174 |
| Ventilation (hours) | 119.24 ± 140.92 | 104.58 ± 128.05 | 117.53 ± 141.42 | 117.04 ± 145.42 | 136.59 ± 146.05 | 3.38 | 0.018 |
| Comorbidity | |||||||
| Hypertension (n(%)) | 1.00 | 0.802 | |||||
| No | 980 (56.26) | 243 (55.73) | 239 (54.94) | 253 (58.16) | 245 (56.19) | ||
| Yes | 762 (43.74) | 193 (44.27) | 196 (45.06) | 182 (41.84) | 191 (43.81) | ||
| Type 2 diabetes mellitus (n(%)) | 111.24 | < 0.001 | |||||
| No | 1,275 (73.19) | 381 (87.39) | 343 (78.85) | 301 (69.20) | 250 (57.34) | ||
| Yes | 467 (26.81) | 55 (12.61) | 92 (21.15) | 134 (30.80) | 186 (42.66) | ||
| Heart failure (n(%)) | 1.26 | 0.738 | |||||
| No | 1,271 (72.96) | 319 (73.17) | 319 (73.33) | 309 (71.03) | 324 (74.31) | ||
| Yes | 471 (27.04) | 117 (26.83) | 116 (26.67) | 126 (28.97) | 112 (25.69) | ||
| Scoring systems | |||||||
| SOFA score (score) | 6.62 ± 4.19 | 5.64 ± 3.64 | 5.89 ± 3.68 | 6.56 ± 4.09 | 8.39 ± 4.70 | 40.90 | < 0.001 |
| APSIII score (score) | 53.81 ± 24.70 | 48.43 ± 21.65 | 50.89 ± 23.02 | 52.29 ± 23.29 | 63.64 ± 27.70 | 34.32 | < 0.001 |
| SAPSII score (score) | 40.91 ± 15.03 | 38.73 ± 13.23 | 39.88 ± 13.90 | 40.03 ± 14.75 | 45.00 ± 17.21 | 15.37 | < 0.001 |
TyG index: Q1 (Quartile 1; TyG index ≤ 8.56, n = 436), Q2 (Quartile 2; 8.56 < TyG index ≤ 9.03), Q3 (Quartile 3; 9.03 < TyG index ≤ 9.56) and Q4 (Quartile 4; TyG index > 9.56). Continuous variables are expressed as the median and interquartile range. Counting data are presented as numbers and percentages. The medical condition was defined based on the ICD-9 code. TyG, triglyceride-glucose; WBC, white blood cell; RBC, red blood cell; RDW, red blood cell distribution width; HbA1c, glycated hemoglobin A1c; TG, triglycerides; TC, total cholesterol; CRRT, continuous renal replacement therapy; SASPII, simplified acute physiology score II; SOFA, sequential organ failure assessment; F, ANOVA; χ², Chi-square test; SD, standard deviation
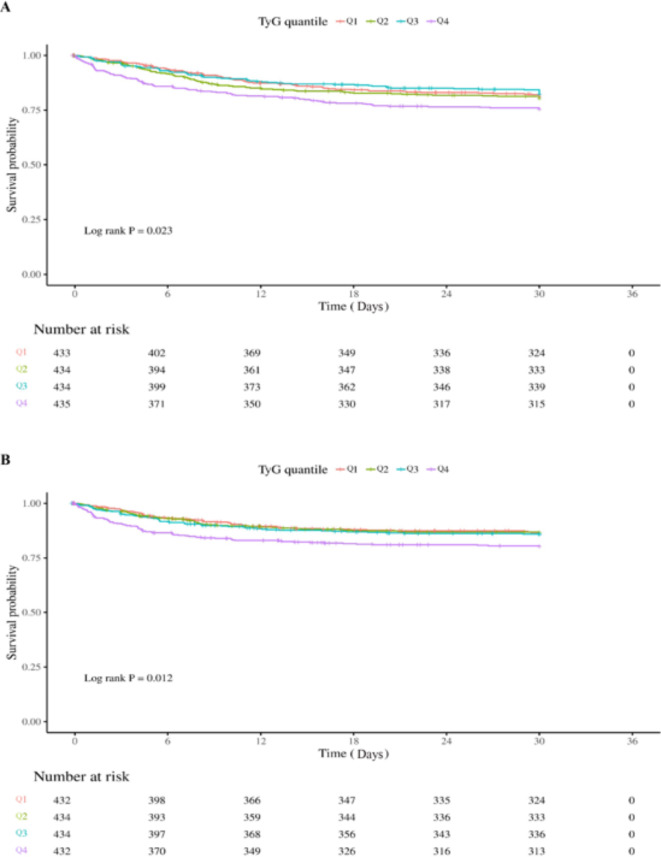
KaplanMeier survival curve analysis
The Kaplan-Meier survival curves, depicted in Fig. 2, demonstrated a progressively deteriorating survival probability for critically ill patients with sepsis from Q1 to Q4. Furthermore, there were evident intergroup differences in mortality rates observed during both ICU admission and hospitalization (log-rank test, P = 0.023, 0.012, respectively) (Fig. 2A and B).
Fig. 2.
Kaplan-Meier survival curve of cumulative survival rate during hospitalization and ICU stay. (A): Kaplan-Meier survival curve of cumulative survival rate during hospitalization. (B): Kaplan-Meier survival curve of cumulative survival rate during ICU stay.
Cox regression models for all-cause mortality (in hospital and ICU)
In the Cox regression analysis, a higher TyG index was positively correlated with increased mortality rates in both the ICU and hospital settings among critically ill patients with sepsis. When the TyG index was analyzed as a continuous variable, it was independently associated with a higher risk of hospital mortality (All P < 0.05). When categorized into quartiles, Model 1 revealed that the risk of hospital mortality for Q4 was 63% higher than for Q1 (HR 1.63 [95% CI 1.22 to 2.18], P < 0.01), whereas no significant differences were noted between Q2, Q3, and Q1.
For ICU mortality, the TyG index, when used as a continuous variable, was significantly associated with an elevated risk of ICU death in Models 1 and 2 (HR 1.26 [95% CI 1.10 to 1.45], P < 0.001; HR 1.19 [95% CI 1.05 to 1.35], P = 0.006, respectively), but it was not associated with changes in ICU mortality risk in Model 3. Furthermore, when the TyG index was categorized into quartiles, Model 1 indicated that the risk of ICU mortality for Q4 was 1.79 times that of Q1 (HR 1.79 [95% CI 1.28 to 2.51], P < 0.001), with no significant differences between Q1, Q2, and Q3. In Model 3, the risk of ICU mortality increased with higher quartiles of the TyG index, with risks for Q2, Q3, and Q4 being 33% (HR 1.33 [95% CI 1.20 to 1.53], P < 0.001), 75% (HR 1.75 [95% CI 1.16 to 2.63], P = 0.007), and 240% (HR 3.40 [95% CI 2.24 to 5.16], P < 0.001) higher than Q1, respectively (Table 2).
Table 2.
The association between TyG index groups and in-hospital and ICU mortality.
| Exposure | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| In-hospital mortality | ||||||
| TyG as continuous | 1.19 (1.05–1.35) | 0.006 | 1.19 (1.05–1.35) | 0.006 | 1.44 (1.16–1.78) | < 0.001 |
| Q1 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | |||
| Q2 | 1.17 (0.86–1.59) | 0.315 | 1.41 (1.05–1.90) | 0.024 | 1.02 (0.68–1.55) | 0.918 |
| Q3 | 1.10 (0.80–1.50) | 0.567 | 1.26 (0.95–1.68) | 0.108 | 0.17 (0.12–0.25) | < 0.001 |
| Q4 | 1.63 (1.22–2.18) | 0.001 | 1.19 (0.91–1.56) | 0.211 | 0.13 (0.09–0.19) | < 0.001 |
| P for trend | ||||||
| ICU mortality | ||||||
| TyG as continuous | 1.26 (1.10–1.45) | 0.001 | 1.19 (1.05–1.35) | 0.006 | 1.19 (0.97–1.46) | 0.099 |
| Q1 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | |||
| Q2 | 1.08 (0.75–1.57) | 0.667 | 1.18 (0.84–1.67) | 0.337 | 1.33 (1.20–1.53) | < 0.001 |
| Q3 | 1.26 (0.88–1.81) | 0.212 | 1.23 (0.89–1.70) | 0.211 | 1.75 (1.16–2.63) | 0.007 |
| Q4 | 1.79 (1.28–2.51) | < 0.001 | 1.06 (0.78–1.44) | 0.690 | 3.40 (2.24–5.16) | < 0.001 |
| P for trend | ||||||
* TyG index: Q1 (Quartile 1; TyG index ≤ 8.56, n = 436), Q2 (Quartile 2; 8.56 < TyG index ≤ 9.03), Q3 (Quartile 3; 9.03 < TyG index ≤ 9.56) and Q4 (Quartile 4; TyG index > 9.56). HR: hazard ratio; CI: confidential interval.
Model 1: Cox univariate analysis
Model 2: Adjusted for age, gender, height, weight, and race
Model 3: Adjusted for age, gender, height, weight, race, insurance, marital status, WBC, RBC, RDW, albumin, chloride, ALT, AST, SOFA score, APSIII score, SAPSII score, OASIS score, Charlson score, Hypertension, Type 2 diabetes mellitus, heart failuremi, malignant tumor, chronic kidney disease, acute renal failure, stroke, hyperlipidemia, chronic obstructive pulmonary disease
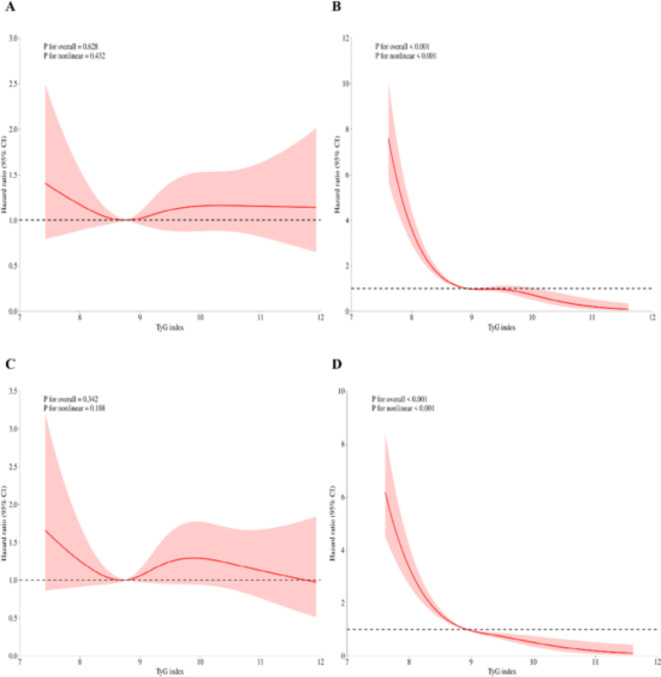
We subsequently employed an RCS regression model to elucidate the risk and discovered a nonlinear association between the TyG index and mortality. Figure 3A and B illustrate the results of the univariate and multivariate analyses regarding the relationship between the TyG index and in-hospital mortality, respectively. Figure 3C and D present the findings of the univariate and multivariate analyses concerning the association between the TyG index and ICU mortality, respectively. Before adjusting for in-hospital mortality, the p-value for the overall effect was 0.628, and the p-value for the nonlinear effect was 0.432. Following adjustment, all p-values were less than 0.001. For ICU mortality, before adjustment, the p-value for the overall effect was 0.342, and the p-value for the nonlinear effect was 0.188. After adjustment, all p-values were below 0.001. Figure 3. demonstrates that the inflection point in both multifactorial models is 8.9.
Fig. 3.
RCS regression for TyG and mortality. (a) RCS regression for in-hospital mortality in univariate analysis. (b) RCS regression for in-hospital mortality in multivariate analysis. (c) RCS regression for ICU mortality in univariate analysis. (d) RCS regression for ICU mortality in multivariate analysis. The p-values presented in the figures were derived from a likelihood ratio test comparing the spline model to the null model. All P-values for nonlinearity were less than 0.001.
Subgroup analysis
In the subgroup analysis, the directionality of effect estimates in subgroups aligned with the overall outcomes. The subgroup analyses were stratified by age, gender, BMI, hypertension, type 2 diabetes mellitus, heart failure, continuous renal replacement therapy, and mechanical ventilation. As depicted in Fig. 4A, before adjusting for covariates, the directional trends of effect estimates in in-hospital mortality were consistent with the overall outcomes in nearly half of the subgroups. Similarly, all subgroups aligned with the overall outcomes in ICU mortality. Furthermore, a significant interaction was observed between the subgroup parameter of mechanical ventilation (P for interaction = 0.032).
Fig. 4.
Forest plots for different subgroup analyses of HRs for the association between TyG index and in-hospital mortality and ICU mortality. (A) Forest plots for different subgroup analysis of HRs for the association between TyG index and in-hospital mortality and ICU mortality before covariates adjustment; (B) Forest plots for different subgroup analysis of HRs for the association between TyG index and in-hospital mortality and ICU mortality after covariates adjustment. BMI, Body Mass Index; diaht, Diagnosed as Hypertension; diadm2, Diagnosed as Diabetes mellitus type 2; diahf, Diagnosed as Heart failure; crrt, Continuous renal replacement therapy; HR, Hazard Risk.
After adjustment for covariates (Fig. 4B), the directionality of effect estimates in in-hospital mortality remained consistent with the overall outcomes across nearly all subgroups, with the exception of those with BMI < 27.4 and no mechanical ventilation. Additionally, the directional trends of effect estimates in ICU mortality were consistent with the overall outcomes across all subgroups. No significant interactions were identified between the TyG index and age, gender, BMI, hypertension, type 2 diabetes mellitus, heart failure, continuous renal replacement therapy, and ventilation (interaction P all > 0.05).
Discussion
Our study retrospectively delved into the correlation between the TyG index and mortality rate in critically ill sepsis patients based on the latest MIMIC database. RCS was also employed to explore the nonlinear relationship between the TyG index and mortality rate. To account for potential confounding variables, Cox regression analysis, merging multiple models, and appropriately classified subgroup analysis were carried out. Our findings indicate that a higher TyG index is associated with increased in-hospital and ICU mortality rates among critically ill sepsis patients. Moreover, there was a non-linear correlation between the TyG index and in-hospital mortality and ICU mortality of critically ill sepsis patients. Furthermore, subgroup analyses were performed to compare hazard risks across different patient demographics and conditions, including age, sex, BMI, hypertension, type 2 diabetes, heart failure, continuous renal replacement therapy, and ventilation. The results showed consistency across these subgroups, with no significant differences, suggesting that these factors did not substantially impact the reliability of the TyG index within our study population. This provides valuable insights into the relationship between glucose and lipid metabolism parameters and clinical outcomes in severe sepsis.
Recent studies from China, South Korea, and Spain have confirmed the predictive value of the TyG index for type 2 diabetes24–26, and it has also been linked to hypertension27, coronary artery stenosis28, hyperuricemia29, and other conditions. Most research has focused on the association between the TyG index and major adverse cardiovascular events. Our study, however, found a significant correlation between a higher TyG index and increased risk of in-hospital and ICU mortality in severe sepsis patients. These results are consistent with findings from Fang et al.15 and Zheng et al.16. By further examining the cumulative survival time of patients with severe sepsis and the nonlinear relationship between the TyG index and outcomes, our study offers additional evidence supporting our hypothesis and provides a more comprehensive understanding of the association between the TyG index and clinical outcomes.
The association between the TyG index and the risk of poor prognosis in sepsis patients may be attributed to disruptions in glucose and lipid metabolism observed in these individuals. The coexistence of hyperglycemia and dyslipidemia during sepsis, and their significant correlation with disease prognosis, underscore the importance of understanding the metabolic disturbances and their metabolites. This may facilitate the identification of early biomarkers with prognostic potential. In sepsis, the body experiences hypercatabolism driven by stress hormones and pro-inflammatory cytokines. Initially, the stress response leads to the secretion of various hormones and neurotransmitters, stimulating the liver to synthesize and release substantial amounts of glycogen into the bloodstream, thereby increasing blood glucose levels30. As the disease advances, enhanced gluconeogenesis results in elevated levels of anaerobic metabolites in the circulation, exacerbating hyperglycemia31. Additionally, the presence of infectious agents may impair pancreatic function, thereby reducing the production of insulin32. Recent studies have highlighted that during sepsis, a substantial release of inflammatory factors occurs. These factors can impair insulin signaling and contribute to insulin resistance through mechanisms involving free fatty acids33, oxidative stress34, and endoplasmic reticulum stress35. Such acute insulin resistance can occur within a few hours after the body’s acute response and last for several weeks36. Similarly, lipids also play an important role in the pro-inflammatory and anti-inflammatory effects of sepsis microvasculature, and changes in lipid metabolism and activation of lipid signaling pathways have been found to be components of the complex environment underlying the pathophysiology of sepsis37,38. During the onset and progression of severe sepsis, triglyceride levels rise more significantly than other lipoproteins. This increase is driven by cytokines, leading to the synthesis and secretion of triglyceride-rich low-density lipoproteins by the liver, a condition clinically referred to as the high-fat state of sepsis. It is now also considered a part of the body’s innate immunity, especially in binding and inactivating lipopolysaccharides (LPS)39. The protective effect of lipoproteins in infected patients is primarily due to their ability to bind and neutralize LPS from gram-negative bacteria and lipophilic acids from gram-positive bacteria. This interaction effectively blocks the biological activity of endotoxins, preventing them from activating inflammatory cells40,41. Animal experiments have shown that after LPS injection or live infection, animals with increased blood lipoprotein concentration have lower levels of cytokines, reduced organ dysfunction, increased LPS clearance, and improved survival rates42–44. In critical cases, lipolysis of adipose tissue can elevate triglyceride levels, particularly when lipid absorption is impaired45. Additionally, in sepsis, increased VLDL synthesis by the liver and decreased VLDL clearance by peripheral tissues exacerbate lipid metabolism disorders46. The mechanism is related to the action of some cytokines (TNF-α, IL-1, IL-6, and INF-γ)47,48 on the liver to increase fatty acid synthesis. Therefore, abnormal glucose and lipid metabolism may be crucial in the progression of sepsis, and the TyG index could potentially serve as a marker for monitoring these metabolic changes and even predicting the prognosis.
A systematic review and meta-analysis by Hofmaenner et al.49 found that in critically ill sepsis patients, lower levels of total cholesterol, high-density lipoprotein cholesterol, and low-density lipoprotein were associated with higher mortality, whereas triglyceride levels did not show a similar association. This finding was corroborated by a clinical study50. In contrast, Muhammad et al.51 and Yin et al.52 highlighted a link between the TyG index and cardiovascular events, while Liu et al.53 reported no association between the TyG index and cardiovascular or all-cause mortality, suggesting contradictory outcomes. Notably, none of these studies specifically focused on critically ill patients with relevant disease conditions. Previous research54,55 indicates that the TyG index may be a more accurate measure of insulin resistance compared to alternatives like the Homeostasis Model Assessment of Insulin Resistance (HOMA-IR). In critical care settings, the TyG index has demonstrated potential prognostic value. A Mendelian randomization study identified a causal relationship between a higher TyG index and reduced stroke risk in critically ill patients56, and Huang et al.57 observed a significant negative correlation between the TyG-BMI index and all-cause mortality in severe stroke cases. These studies suggest a negative correlation between the TyG index and adverse clinical outcomes. In a retrospective study of non-surgical intensive care patients, Nishigoori et al.58 found that a high TyG index was correlated with negative outcomes in patients with acute coronary syndrome, but also found that a low TyG index was associated with adverse outcomes in acute heart failure. It seems that the relationship between high or low TyG index and clinical outcomes depends on specific outcome types under specific disease conditions. However, more studies have reported a positive relationship between high TyG index and poor prognosis in critically ill patients. For example, Liu et al.59 and Yang et al.60 both found that a high TyG index correlates with increased mortality in critically ill patients with cerebral hemorrhage. Although findings are not entirely consistent, they underscore the potential of the TyG index in predicting mortality among critically ill patients. Additionally, a review61 noted persistent lipid abnormalities in sepsis survivors and highlighted the role of these factors in atherosclerosis, which may contribute to mortality during hospitalization or ICU admission. Thus, the TyG index, integrating indicators of lipid and glucose metabolism, may serve as a valuable marker for insulin resistance and a predictor of metabolic abnormalities in acute sepsis.
This study offers therapeutic insights into the management of critical sepsis and advocates for a comprehensive risk management strategy to mitigate sepsis-related mortality associated with elevated TyG index levels. Such a strategy should include active management of cardiovascular risk factors such as lipid levels, body mass index (BMI), fasting blood glucose, and glycosylated hemoglobin. Regular monitoring and timely intervention for ICU patients with elevated TyG index levels are essential to reduce adverse outcomes. While the “gold standard” for insulin assessment is the hyperinsulinemic-euglycemic clamp test, its extensive duration, complexity, and high cost limit its routine clinical application. In contrast, the TyG index, which relies on simple laboratory biochemical markers, offers a more economical and time-efficient alternative. This suggests that the TyG index has the potential to evolve into a straightforward and effective tool for monitoring sepsis prognosis. Dynamic assessment of the TyG index in clinical practice could facilitate real-time evaluation of ICU sepsis patients’ prognoses, allowing for more tailored and cautious treatment strategies in cases of identified high mortality risk. Additionally, the TyG index may serve as a valuable evaluation tool for assessing the efficacy of treatments targeting glucose and lipid metabolism disorders in sepsis patients. Continuous monitoring of the TyG index and analysis of its trends can aid in the adjustment of therapeutic interventions for glucose and lipid management.
However, this study has several limitations. The study cohort, derived from the MIMIC-IV database, provides a large sample size and reliable results, yet the critically ill population studied may not fully represent a continuous spectrum of metabolic status. Residual confounding remains a possibility within the model, and specific causes of patient mortality are not accessible. Moreover, the exclusion of certain laboratory test data due to missing values may introduce bias. Lastly, this study represents a retrospective and observational investigation; therefore, it cannot definitively establish a causal relationship between the TyG index and mortality in patients with severe sepsis. Future research of a higher evidence level is needed to validate the association between the TyG index and clinical outcomes in septic patients.
Conclusions
In conclusion, an elevated TyG index is associated with a higher risk of in-hospital and ICU mortality in critically ill sepsis patients. Our analysis demonstrated a nonlinear relationship that remained consistent across subgroups, thereby enhancing the stability and robustness of our findings. Moreover, this study, with its comprehensive statistical analyses and larger patient cohort in comparison to previous investigations, clearly quantifies the heightened mortality risk correlated with the TyG index. Our research underscores the utility of the TyG index as a crucial biomarker for clinicians and its role in facilitating early identification and intervention for high-risk sepsis patients in the ICU. Therefore, the foregoing results may lay a foundation for future prospective studies aimed at validating these observations and evaluating potential interventions based on the TyG index to improve sepsis patient outcomes.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Abbreviations
- ICU
Intensive care units
- TG
Triglyceride
- HDL-C
High-density lipoprotein cholesterol
- BMI
Body Mass Index
- TyG
Triglyceride-lucose
- MIMIC-IV
Medical information mart for intensive care IV
- SOFA
Sequential organ failure assessment
- RCS
Restricted cubic splines
- COPD
Chronic obstructive pulmonary disease
- CKD
Chronic kidney disease
- HR
Hazard risk
- CI
Confidence interval
- HF
Heart failure
- HT
Hypertension
- DM2
Diabetes mellitus type 2
- OASIS
Oxford acute severity of illness score
- SAPS II
Simplified acute physiology score II
- WBC
White blood cell
- Rbc
Red blood cell
- RDW
Red blood cell distribution width. AKI, acute kidney injury
- CRRT
Continuous renal replacement therapy
- HLP
Hyperlipidemia
- TB
Tuberculosis
- arf
acute renal failure
- MT
Malignant tumor
- MI
Myocardial infarction
Author contributions
JQ L, ZY X and XY Z contributed equally to this work. JQ L and N H designed and conceptualized the study. JQ L, ZY X, XY Z, JY S, JL L, SY C and GY J completed the record retrieval and data extraction. Mathematical modeling and meta-analysis were conducted with the help of YF F and JY S. The original draft was written by JQ L, ZY X and XY Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the HwaMei Research Foundation of Ningbo No.2 Hospital (Grant No.2022HMKY48 and No.2023HMZD07), Medical Scientific Research Foundation of Zhejiang Province (Grant No.2023RC081), the Project of NINGBO Leading Medical & Health Discipline (Project No.: 2022-F17), and the Ningbo Top Medical and Health Research Program (No.2023030615). Funders played no role in the study design, execution, or manuscript writing.
Data availability
Raw data supporting the obtained results are available at the corresponding author.
Declarations
Ethics approval and consent to participate
This study adhered to the principles outlined in the Declaration of Helsinki. Furthermore, it is important to note that the MIMIC-IV database received approval from the Massachusetts Institute of Technology and the Beth Israel Deaconess Medical Center. The copies of the datasets used in this study are available from the MIMIC database. Due to the retrospective nature of the study, the need of informed consent was waived by the Human Research Ethics Committee of Ningbo No.2 Hospital.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jiaqi Lou, Ziyi Xiang and Xiaoyu Zhu contributed equally to this work.
References
- 1.Wang, L., Wang, M., Du, J. & Gong, Z. C. Intensive insulin therapy in sepsis patients: Better data enables better intervention. Heliyon. 9(3), e14063. 10.1016/j.heliyon.2023.e14063 (2023). Published 2023 Feb 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.See, K. C. Glycemic targets in critically ill adults: A mini-review. World J. Diabetes. 12(10), 1719–1730. 10.4239/wjd.v12.i10.1719 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang, Y. H., Wen, R., Yang, N., Zhang, T. N. & Liu, C. F. Roles of protein post-translational modifications in glucose and lipid metabolism: Mechanisms and perspectives. Mol. Med.29(1), 93. 10.1186/s10020-023-00684-9 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Margalin, B., Arfijanto, M. V. & Hadi, U. Effector function and neutrophil cell death in the severity of sepsis with diabetes mellitus. Narra J.4(1), e532. 10.52225/narra.v4i1.532 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosendo-Silva, D., Viana, S., Carvalho, E., Reis, F. & Matafome, P. Are gut dysbiosis, barrier disruption, and endotoxemia related to adipose tissue dysfunction in metabolic disorders? Overview of the mechanisms involved. Intern. Emerg. Med.18(5), 1287–1302. 10.1007/s11739-023-03262-3 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor, R. et al. Low circulatory levels of total cholesterol, HDL-C and LDL-C are associated with death of patients with sepsis and critical illness: Systematic review, meta-analysis, and perspective of observational studies. EBioMedicine. 100, 104981. 10.1016/j.ebiom.2024.104981 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.von Eckardstein, A., Nordestgaard, B. G., Remaley, A. T. & Catapano, A. L. High-density lipoprotein revisited: Biological functions and clinical relevance. Eur. Heart J.44(16), 1394–1407. 10.1093/eurheartj/ehac605 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stoudt, K. & Chawla, S. Don’t sugar coat it: Glycemic control in the intensive care unit. J. Intensive Care Med.34(11–12), 889–896. 10.1177/0885066618801748 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiao, M. et al. U-shaped association between serum triglyceride levels and mortality among septic patients: An analysis based on the MIMIC-IV database. PLoS ONE. 18(11), e0294779. 10.1371/journal.pone.0294779 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sánchez-García, A. et al. Diagnostic accuracy of the triglyceride and glucose index for insulin resistance: A systematic review. Int. J. Endocrinol.2020(4678526). 10.1155/2020/467852 (2020). [DOI] [PMC free article] [PubMed]
- 11.Yin, J. L. et al. Triglyceride-glucose index and health outcomes: An umbrella review of systematic reviews with meta-analyses of observational studies. Cardiovasc Diabetol. 23(1), 177 (2024). 10.1186/s12933-024-02241- [DOI] [PMC free article] [PubMed]
- 12.Behnoush, A. H. et al. The importance of assessing the triglyceride-glucose index (TyG) in patients with depression: A systematic review. Neurosci. Biobehav Rev.159, 105582. 10.1016/j.neubiorev.2024.105582 (2024). [DOI] [PubMed] [Google Scholar]
- 13.Fang, Y. et al. Triglyceride-glucose index predicts sepsis-associated acute kidney injury and length of stay in sepsis: A MIMIC-IV cohort study. Heliyon. 10(7), e29257. 10.1016/j.heliyon.2024.e29257 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng, R. et al. Association between triglyceride-glucose index and in-hospital mortality in critically ill patients with sepsis: Analysis of the MIMIC-IV database. Cardiovasc. Diabetol.22(1), 307. 10.1186/s12933-023-02041-w (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prescott, H. C. & Ostermann, M. What is new and different in the 2021 surviving Sepsis Campaign guidelines. Was ist neu und was ist anders in den SSC(surviving Sepsis Campaign)-Leitlinien. Med. Klin. Intensivmed Notfmed. 118(Suppl 2), 75–79. 10.1007/s00063-023-01028-5 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Briegel, J. & Möhnle, P. Surviving Sepsis Campaign Update 2018: Das 1–h-Bundle: Hintergrund zu den neuen empfehlungen [Surviving Sepsis Campaign update 2018: The 1 h bundle : background to the new recommendations]. Anaesthesist. 68(4), 204–207. 10.1007/s00101-019-0571-5 (2019). [DOI] [PubMed] [Google Scholar]
- 17.Nazareth, T. A. et al. Patients with idiopathic membranous nephropathy: A real-world clinical and economic analysis of U.S. Claims Data. J. Manag. Care Spec. Pharm.25(9), 1011–1020. 10.18553/jmcp.2019.18456 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seckel, M. A. Sepsis best practices: Definitions, guidelines, and updates. Nursing. 54(6), 31–39. 10.1097/NSG.0000000000000010 (2024). [DOI] [PubMed] [Google Scholar]
- 19.Alcamo, A. M. et al. Diagnostic identification of acute brain dysfunction in pediatric sepsis and septic shock in the electronic health record: A comparison of four definitions in a reference dataset. Pediatr. Crit. Care Med.1310.1097/PCC.0000000000003529 (2024). [DOI] [PMC free article] [PubMed]
- 20.Pérez-Tome, J. C., Parrón-Carreño, T., Castaño-Fernández, A. B., Nievas-Soriano, B. J. & Castro-Luna, G. Sepsis mortality prediction with machine learning tecniques. Med Intensiva (Engl Ed). Published Online June. 13. 10.1016/j.medine.2024.05.009 (2024). [DOI] [PubMed]
- 21.Yang, B. et al. Effects of Ondansetron exposure during ICU stay on outcomes of critically ill patients with sepsis: A cohort study. Front. Cell. Infect. Microbiol.13, 1256382. 10.3389/fcimb.2023.1256382 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu, X., Xin, Q., Hao, Y., Zhang, J. & Ma, T. An early warning model for predicting major adverse kidney events within 30 days in sepsis patients. Front. Med. (Lausanne). 10, 1327036. 10.3389/fmed.2023.1327036 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.da Silva, A., Caldas, A. P. S., Rocha, D. M. U. P. & Bressan, J. Triglyceride-glucose index predicts independently type 2 diabetes mellitus risk: A systematic review and meta-analysis of cohort studies. Prim. Care Diabetes. 14(6), 584–593. 10.1016/j.pcd.2020.09.001 (2020). [DOI] [PubMed] [Google Scholar]
- 24.Navarro-González, D., Sánchez-Íñigo, L., Pastrana-Delgado, J., Fernández-Montero, A. & Martinez, J. A. Triglyceride-glucose index (TyG index) in comparison with fasting plasma glucose improved diabetes prediction in patients with normal fasting glucose: the vascular-metabolic CUN cohort. Prev. Med.86, 99–105. 10.1016/j.ypmed.2016.01.022 (2016). [DOI] [PubMed] [Google Scholar]
- 25.Lee, S. H. et al. Predicting the development of diabetes using the product of triglycerides and glucose: The Chungju Metabolic Disease Cohort (CMC) study. PLoS ONE. 9(2), e90430. 10.1371/journal.pone.0090430 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang, M. et al. Cumulative increased risk of incident type 2 diabetes mellitus with increasing triglyceride glucose index in normal-weight people: The rural Chinese cohort study. Cardiovasc. Diabetol.16(1), 30. 10.1186/s12933-017-0514-x (2017). Published 2017 Mar 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang, C., Song, Y. & Wang, P. Relationship between triglyceride-glucose index and new-onset hypertension in general population-a systemic review and meta-analysis of cohort studies. Clin. Exp. Hypertens.46(1), 2341631. 10.1080/10641963.2024.2341631 (2024). [DOI] [PubMed] [Google Scholar]
- 28.Darroudi, S. et al. Triglyceride glucose index and triglyceride HDL ratio as predictors of coronary artery stenosis in diabetic and non-diabetic patients. Nutr. Metab. Cardiovasc. Dis.34(7), 1692–1695. 10.1016/j.numecd.2023.12.001 (2024). [DOI] [PubMed] [Google Scholar]
- 29.Wang, L. et al. A national study exploring the association between triglyceride-glucose index and risk of hyperuricemia events in adults with hypertension. Prev. Med. Rep.43, 102763. 10.1016/j.pmedr.2024.102763 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Srdić, T. et al. From Molecular mechanisms to clinical therapy: Understanding Sepsis-Induced multiple organ dysfunction. Int. J. Mol. Sci.25(14), 7770. 10.3390/ijms25147770 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nofal, M. A. et al. Recent trends in septic shock management: A narrative review of current evidence and recommendations. Annals Med. Surg. (2012). 86(8), 4532–4540. 10.1097/MS9.0000000000002048 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Plata-Menchaca, E. P., Ruiz-Rodríguez, J. C. & Ferrer, R. Early diagnosis of Sepsis: The role of biomarkers and Rapid Microbiological tests. Semin. Respir. Crit. Care Med.45(4), 479–490. 10.1055/s-0044-1787270 (2024). [DOI] [PubMed] [Google Scholar]
- 33.Gasparini, S. J. et al. Androgens sensitise mice to glucocorticoid-induced insulin resistance and fat accumulation. Diabetologia. 62(8), 1463–1477. 10.1007/s00125-019-4887-0 (2019). [DOI] [PubMed] [Google Scholar]
- 34.Gupta, R. A., Motiwala, M. N., Dumore, N. G., Danao, K. R. & Ganjare, A. B. Effect of piperine on inhibition of FFA induced TLR4 mediated inflammation and amelioration of acetic acid induced ulcerative colitis in mice. J. Ethnopharmacol.164, 239–246. 10.1016/j.jep.2015.01.039 (2015). [DOI] [PubMed] [Google Scholar]
- 35.Degirmenci, I. et al. Common variants of genes encoding TLR4 and TLR4 pathway members TIRAP and IRAK1 are effective on MCP1, IL6, IL1β, and TNFα levels in type 2 diabetes and insulin resistance. Inflamm. Res.68(9), 801–814. 10.1007/s00011-019-01263-7 (2019). [DOI] [PubMed] [Google Scholar]
- 36.Nejati, M., Abbasi, S., Farsaei, S. & Shafiee, F. L-carnitine supplementation ameliorates insulin resistance in critically ill acute stroke patients: A randomized, double-blinded, placebo-controlled clinical trial. Res. Pharm. Sci.17(1), 66–77. 10.4103/1735-5362.329927 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Golucci, A. P. B. S., Marson, F. A. L., Ribeiro, A. F. & Nogueira, R. J. N. Lipid profile associated with the systemic inflammatory response syndrome and sepsis in critically ill patients. Nutrition. 55–56, 7–14. 10.1016/j.nut.2018.04.007 (2018). [DOI] [PubMed] [Google Scholar]
- 38.Hofmaenner, D. A., Kleyman, A., Press, A., Bauer, M. & Singer, M. The many roles of cholesterol in Sepsis: A review. Am. J. Respir Crit. Care Med.205(4), 388–396. 10.1164/rccm.202105-1197TR (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barcia, A. M. & Harris, H. W. Triglyceride-rich lipoproteins as agents of innate immunity. Clin. Infect. Dis.41(Suppl 7), S498–S503. 10.1086/432005 (2005). [DOI] [PubMed] [Google Scholar]
- 40.Wu, A., Hinds, C. J. & Thiemermann, C. High-density lipoproteins in sepsis and septic shock: metabolism, actions, and therapeutic applications. Shock. 21(3), 210–221. 10.1097/01.shk.0000111661.09279.82 (2004). [DOI] [PubMed] [Google Scholar]
- 41.Grin, P. M. et al. Low-density lipoprotein (LDL)-dependent uptake of Gram-positive lipoteichoic acid and Gram-negative lipopolysaccharide occurs through LDL receptor. Sci. Rep. 8(1), 10496 (2018). 10.1038/s41598-018-28777-0 [DOI] [PMC free article] [PubMed]
- 42.McDonald, M. C. et al. Reconstituted high-density lipoprotein attenuates organ injury and adhesion molecule expression in a rodent model of endotoxic shock. Shock. 20(6), 551–557. 10.1097/01.shk.0000097249.97298.a3 (2003). [DOI] [PubMed] [Google Scholar]
- 43.Guo, L. et al. High density lipoprotein protects against polymicrobe-induced sepsis in mice. J. Biol. Chem.288(25), 17947–17953. 10.1074/jbc.M112.442699 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee, R. P. et al. High-density lipoprotein prevents organ damage in endotoxemia. Res. Nurs. Health. 30(3), 250–260. 10.1002/nur.20187 (2007). [DOI] [PubMed] [Google Scholar]
- 45.Green, P., Theilla, M. & Singer, P. Lipid metabolism in critical illness. Curr. Opin. Clin. Nutr. Metab. Care. 19(2), 111–115. 10.1097/MCO.0000000000000253 (2016). [DOI] [PubMed] [Google Scholar]
- 46.Pirillo, A., Catapano, A. L. & Norata, G. D. HDL in infectious diseases and sepsis. Handb. Exp. Pharmacol.224, 483–508. 10.1007/978-3-319-09665-0_15 (2015). [DOI] [PubMed] [Google Scholar]
- 47.Bartolomé, N., Arteta, B., Martínez, M. J., Chico, Y. & Ochoa, B. Kupffer cell products and interleukin 1beta directly promote VLDL secretion and apoB mRNA up-regulation in rodent hepatocytes. Innate Immun.14(4), 255–266. 10.1177/1753425908094718 (2008). [DOI] [PubMed] [Google Scholar]
- 48.Korpi, F. et al. Flagellin and pilin immunization against multi-drug resistant Pseudomonas aeruginosa protects mice in the burn wound sepsis model. Immunol. Lett.176, 8–17. 10.1016/j.imlet.2016.04.002 (2016). [DOI] [PubMed] [Google Scholar]
- 49.Hofmaenner, D. A. et al. Association between Hypocholesterolemia and Mortality in critically ill patients with Sepsis: A systematic review and Meta-analysis. Crit. Care Explor.5(2), e0860. 10.1097/CCE.0000000000000860 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nabavi, A., Allami, A. & QasemiBarqi, R. Changes in plasma lipid and in-hospital deaths in patients with sepsis. Med. J. Islam Repub. Iran.34, 45. 10.34171/mjiri.34.45 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sardar, M. B. et al. Environmental heavy metal exposure and associated cardiovascular diseases in Light of the triglyceride glucose index. Cardiovasc. Toxicol.10.1007/s12012-024-09913-x (2024). [DOI] [PubMed] [Google Scholar]
- 52.Yin, J. L. et al. Triglyceride-glucose index and health outcomes: An umbrella review of systematic reviews with meta-analyses of observational studies. Cardiovasc. Diabetol.23(1), 177. 10.1186/s12933-024-02241-y (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu, X. et al. Relationship between the triglyceride-glucose index and risk of cardiovascular diseases and mortality in the general population: A systematic review and meta-analysis. Cardiovasc. Diabetol.21(1), 124. 10.1186/s12933-022-01546-0 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guerrero-Romero, F. et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J. Clin. Endocrinol. Metab.95(7), 3347–3351. 10.1210/jc.2010-0288 (2010). [DOI] [PubMed] [Google Scholar]
- 55.Breusing, N. et al. Influence of energy balance and glycemic index on metabolic endotoxemia in healthy men. J. Am. Coll. Nutr.36(1), 72–79. 10.1080/07315724.2016.1156036 (2017). [DOI] [PubMed] [Google Scholar]
- 56.Jiang, Y. et al. Association of triglyceride glucose index with stroke: From two large cohort studies and mendelian randomization analysis. Int. J. Surg. Published Online June. 19. 10.1097/JS9.0000000000001795 (2024). [DOI] [PMC free article] [PubMed]
- 57.Huang, Y., Li, Z. & Yin, X. Long-term survival in stroke patients: Insights into triglyceride-glucose body mass index from ICU data. Cardiovasc. Diabetol.23(1), 137. 10.1186/s12933-024-02231-0 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nishigoori, S. et al. Clinical significance of the triglyceride-glucose index in patients requiring nonsurgical intensive care. Int. Heart J.65(2), 180–189. 10.1536/ihj.23-409 (2024). [DOI] [PubMed] [Google Scholar]
- 59.Liu, D., Ren, B., Tian, Y., Chang, Z. & Zou, T. Association of the TyG index with prognosis in surgical intensive care patients: data from the MIMIC-IV. Cardiovasc. Diabetol.23(1), 193. 10.1186/s12933-024-02293-0 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang, Y. et al. Triglyceride-glucose index as a potential predictor for in-hospital mortality in critically ill patients with intracerebral hemorrhage: A multicenter, case-control study. BMC Geriatr.24(1), 385. 10.1186/s12877-024-05002-4 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Laudanski, K. Persistence of lipoproteins and cholesterol alterations after Sepsis: Implication for atherosclerosis progression. Int. J. Mol. Sci.22(19), 10517. 10.3390/ijms221910517 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data supporting the obtained results are available at the corresponding author.