Abstract
Background
High Neutrophil-to-Lymphocyte Ratio (NLR), Monocyte-to-Lymphocyte Ratio (MLR), Platelet-to-Lymphocyte Ratio (PLR) were associated with worse prognosis of patients with sepsis. In-hospital mortality has been reported to be higher in patients with coronary artery disease (CAD) and sepsis than those with sepsis alone. However, the relationship between NLR, MLR, PLR and mortality in septic patients with coronary artery disease (CAD) remains unclear. The study aimed to explore the association between NLR, MLR, PLR and 28-day all-cause mortality in septic patients with CAD.
Methods
We performed an observational cohort study of septic patients with CAD from the Medical Information Mart for Intensive Care (MIMIC)-IV database between 2008 and 2019. The patients were categorized by three group (Q1: low levels, Q2: medium levels, Q3: high levels) based on tertiles of NLR, MLR, and PLR. The associations between NLR, MLR, PLR and 28-day all-cause mortality were examined using the Cox proportional hazards model. Subsequently, we applied receiver operating characteristic (ROC) analysis for predicting 28-day mortality in septic patients with CAD by combining NLR, MLR and PLR with the modified sequential organ failure assessment (mSOFA) scores.
Results
Overall 1,175 septic patients with CAD were included in the study. Observed all-cause mortality rates in 28 days were 27.1%. Multivariate Cox proportional hazards regression analysis results showed that 28-day all-cause mortality of septic patients with CAD was significantly related to rising NLR levels (adjusted hazard ratio [aHR]: 1.02; 95% confidence interval [CI]: 1.01–1.02; P < 0.001), MLR levels (aHR: 1.29; 95%CI: 1.18–1.41; P < 0.001), and PLR levels (aHR: 1.0007; 95%CI: 1.0004–1.0011; P < 0.001). Meanwhile, the higher levels (Q3) group of NLR, MLR, and PLR also had a higher risk of 28-day all-cause mortality than the lower (Q1) group. The area under the ROC curve of NLR, MLR, PLR, and mSOFA score were 0.630 (95%CI 0.595–0.665), 0.611 (95%CI 0.576–0.646), 0.601 (95%CI 0.567–0.636) and 0.718 (95%CI 0.689–0.748), respectively. Combining NLR, MLR, and PLR with mSOFA scores may improve ability of predicting 28-day mortality (AUC: 0.737, 95%CI 0.709–0.766).
Conclusion
Higher levels of NLR, MLR and PLR were associated with 28-day all-cause mortality in septic patients with CAD. Further investigation will be needed to improve understanding of the pathophysiology of this relationship.
Keywords: Biomarker, Coronary artery disease, Sepsis, Sequential organ failure assessment scores
Introduction
Sepsis is the leading cause of mortality in intensive care units (ICUs), responsible for approximately 30–50% of deaths [1, 2]. Despite the pathophysiology of sepsis remains unclear, organ damage induced by inflammation is well recognized [3, 4]. The cardiovascular system is especially vulnerable to damage during sepsis, and decreased cardiac function has been linked to heightened inflammation, oxidative stress, apoptosis, and restricted autophagy in sepsis [5]. Moreover, heightened endothelial injury has been observed in septic patients with coronary artery disease (CAD) compared to those without CAD [3]. Additionally, a large-scale, real-world cohort study involving over 2.6 million individuals revealed that acute myocardial infarction (AMI) contributed to approximately 4.5% of deaths and was linked to higher mortality rates in patients with sepsis [6]. The recent emphasis of ICU management has been on septic patients with CAD, but the early identification of the poor prognosis associated with these specially subgroup disease remains a challenge for ICU clinicians [7].
Clinical biomarkers derived from laboratory test results have recently played a pivotal role in objectively evaluating disease severity and forecasting clinical prognosis in patients [8–10]. Blood routine examination and biochemistry have garnered considerable attention due to their inclusion as routine test parameters. Neutrophil-to-lymphocyte ratio (NLR), monocyte-to-lymphocyte ratio (MLR), and platelet-to-lymphocyte ratio (PLR) are commonly utilized inflammatory biomarkers easily obtained from blood routine and biochemistry to evaluate inflammatory activity [11–15]. Inflammatory biomarkers have been demonstrated to predict mortality risk in patients with sepsis [16]. For instance, NLR and PLR have been identified as predictors of 90-day mortality in patients with sepsis [17]. Some meta-analysis showed higher NLR and PLR values may indicate unfavorable prognosis in patients with sepsis [18–20]. In patients with CAD, several studies have indicated a correlation between these markers and the severity, prognosis, and presence of CAD [11, 21]; Elevated NLR has been linked to mortality in patients with AMI [22]. Post-AMI patients who do not undergo percutaneous coronary intervention (PCI) may demonstrate an increased inflammatory response and elevated NLR and PLR levels, which are linked to decreased left ventricular thrombus (LVT) resolution despite anticoagulant therapy [23]. Nevertheless, the associations between NLR, MLR, PLR and mortality in septic patients with CAD remains unclear. Additionally, the modified Sequential Organ Failure Assessment (mSOFA) score, as a rapid and comprehensive assessment of sepsis-related organ failure, has been linked to mortality in septic patients [24]. Nonetheless, the predictive value of NLR, MLR, and PLR in combination with mSOFA scores for 28-day mortality in septic patients with CAD is still uncertain.
Hence, the main objectives of this study were : (1) to examine the relationship between NLR, MLR, PLR and 28-day all-cause mortality in septic patients with CAD; and (2) to employ receiver-operator characteristic (ROC) curves to assess the predictive ability of combining NLR, MLR and PLR with mSOFA scores in predicting the 28-day all-cause mortality in septic patients with CAD.
Methods
Study design and population
The study data were based on the Medical Information Mart for Intensive Care (MIMIC-IV 2.2 version) database provided information on 315,460 inpatients of Beth Israel Deaconess Medical Center from 2008 to 2019 for the American cohort [25]. Patients were included meeting the criteria as following: (1) Patients were diagnosed as sepsis within 12 h before or 24 h after ICU admission [26]. (2) combined with CAD; (3) Blood routine and biochemistry measured at ICU admission within 6 h before or 24 h after ICU admission. While patients meeting the following criteria were excluded: (1) insufficient or missing important demographic information and laboratory results (Body Mass Index [BMI], NLR, MLR and PLR); (2) be diagnosed as hematological disease.
To safeguard patient privacy, all personally identifiable information has been anonymized. As all patient records in the MIMIC-IV database were thoroughly de-identified, the need for individual patient consent was deemed unnecessary by the institutional review board of the Beth Israel Deaconess Medical Center, and the study was conducted out in conformity with the Declaration of Helsinki.
Data collection
To access the database, we had gone through a “CITI Data or Specimens Only Research” training course on the National Institutes of Health website, the author was approved to extract data from this database for research purposes (Certificate No: 40,416,369). We extracted variables including demographics, comorbidities, laboratory results, laboratory indices, and discharge status from MIMIC-IV database. Diabetes was defined based on ICD-9 codes 250.0x-250.9x and ICD-10 codes E10-E14. The code for data query and extraction was available from the Repository (website: https://github.com/MIT-LCP/mimic-code). Sepsis was defined according to The Third International Consensus Definitions for Sepsis and Septic Shock [27]. In brief, patients with documented or suspected infection and an acute change in total SOFA score of ≥ 2 points were considered to have sepsis [28]. The code used to extract sepsis patients who meet the sepsis 3.0 standard can be found at https://github.com/alistairewj/sepsis3-mimic. CAD was defined according to the codes 410–411 (ICD-9) and I20-I21 (ICD-10) (https://icd.who.int/browse10/2019/en) [29–32] from disease history and medical record diagnosis, including previous myocardial infarction, or acute coronary syndrome etc. Only the initial admission was taken into account if a patient had multiple admissions.
Definition of NLR, MLR, and PLR
Laboratory test parameters included neutrophil count, monocyte count, lymphocyte count and platelet count. We applied for the average value of laboratory tests in the range from 6 h before admission to 24 h after admission to ICU. Definitions of NLR, MLR, and PLR were as following [11]:
NLR—neutrophil/lymphocyte ratio;
PLR—platelet/lymphocyte ratio;
MLR—monocyte/lymphocyte ratio;
The patients were categorized by three group (Q1: low levels, Q2: medium levels, Q3: high levels) based on triplicate of NLR (Q1 < 5.86, 5.86 ≤ Q2 < 12.58, and Q3 ≥ 12.58), MLR (Q1 < 0.32, 0.32 ≤ Q2 < 0.70, and Q3 ≥ 0.70), and PLR (Q1 < 1.90, 1.90 ≤ Q2 < 90.21, and Q3 ≥ 90.21). The end points of the study included all-cause mortality at 7, 14, 21 and 28 days.
Statistical analysis
SPSS statistical version 26.0 (IBM Corp. Armonk, NY, USA) and R software (version 4.2.1) was performed for the statistical analysis. Continuous variables having a normal distribution were presented as the mean standard deviation (SD), whereas those without a normal distribution were described as the median [interquartile range (IQR)]. Student’s t-test, Wilcoxon rank sum test, and Chi-square test were used to evaluate baseline characteristics and inflammatory biomarkers, while the Kaplan-Meier technique with the log-rank test was used to assess 28-day all-cause mortality. The relationship between NLR, MLR, PLR and 28-day mortality in septic patients with CAD was examined using the Cox proportional hazards model, adjusting for age, gender, BMI, race, cardiovascular disease, chronic pulmonary disease, liver disease, malignancy, dementia, mechanical ventilation. We applied receiver operating characteristic (ROC) analysis for predicting 28-day all-cause mortality in septic patients with CAD by combining NLR, MLR and PLR with mSOFA scores. The Delong’s test was performed to compare different ROC curves. P < 0.05 was considered statistically significant.
Results
Patient characteristics
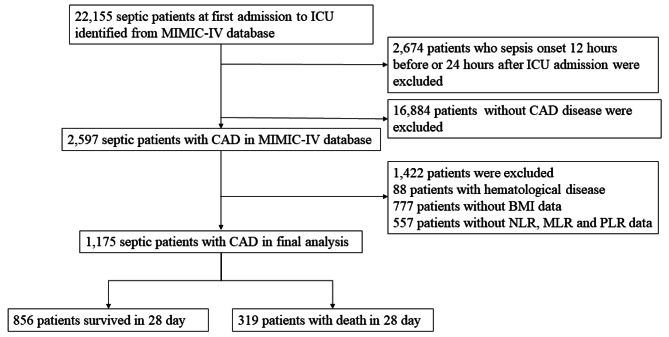
The flow chart of inclusion and exclusion are shown in Fig. 1. This study included 22,155 patients with sepsis from the MIMIC-IV database. 2,674 patients who were identified as sepsis 12 h before or 24 h after ICU admission were excluded; 16,884 patients without CAD disease were excluded. Then 1,422 patients due to hematological disease and the lack of important demographic information and laboratory results were excluded. Finally, a total of 1,175 septic patients with CAD were included in this study. The mean age of overall patients was 71.46 (62.32–79.65) years, and 63.7% of patients were male. Among the 1,175 patients, 856 (72.9%) survived 28 days, while 319 (27.1%) died within 28 days.
Fig. 1.
The flow chart of inclusion and exclusion
Table 1 shows the baseline characteristics of the survival and non-survival groups. Compared with the survival group, the non-survival group was older (75.39 [66.28, 83.76] vs. 69.99 [61.66, 78.53], P < 0.001), and had a lower BMI (27.09 [23.54, 32.26] vs. 28.37 [24.81, 32.67], P < 0.001), higher proportion of liver disease (18.2% vs. 9.5%, P < 0.001), malignancy (11.9% vs. 6.1%, P = 0.001), higher charlson comorbidity Index (CCI) (8 [6–9] vs. 7 [5–8], P < 0.001) and mSOFA scores (6 [4–8] vs. 4 [2–6], P < 0.001). No differences between race and other comorbidities (cardiovascular disease, chronic pulmonary disease, renal disease, diabetes, dementia, and acquired Immunodeficiency Syndrome [AIDS]) were observed in both groups (P > 0.05).
Table 1.
Baseline characteristics
| Variable | Total (n = 1175) |
Survival (n = 856) |
Non-survival (n = 319) |
p |
|---|---|---|---|---|
| Age (y) | 71.46 (62.32, 79.65) | 69.99 (61.66, 78.53) | 75.39 (66.28, 83.76) | < 0.001 |
| Gender (%) | 0.031 | |||
| Female | 426 (36.3) | 294 (34.3) | 132 (41.4) | |
| Male | 749 (63.7) | 562 (65.7) | 187 (58.6) | |
| Race (%) | 0.199 | |||
| White | 754 (64.2) | 566 (66.1) | 188 (58.9) | |
| Black | 77 (6.6) | 53 (6.2) | 24 (7.5) | |
| Asian | 29 (2.5) | 20 (2.3) | 9 (2.8) | |
| Hispanic | 29 (2.5) | 22 (2.6) | 7 (2.2) | |
| Other | 286 (24.3) | 195 (22.8) | 91 (28.5) | |
| BMI (kg/m2) | 27.92 (24.47, 32.45) | 28.37 (24.81, 32.67) | 27.09 (23.54, 32.26) | 0.006 |
| CCI, [M(IQR)] | 7.00 (5.00, 9.00) | 7.00 (5.00, 8.00) | 8.00 (6.00, 9.00) | < 0.001 |
| Comorbidities, n (%) | ||||
| Cardiovascular disease | 753 (64.1) | 540 (63.1) | 213 (66.8) | 0.270 |
| Chronic pulmonary disease | 362 (30.8) | 251 (29.3) | 111 (34.8) | 0.083 |
| Liver disease | 139 (11.8) | 81 (9.5) | 58 (18.2) | < 0.001 |
| Renal disease | 356 (30.3) | 253 (29.6) | 103 (32.3) | 0.404 |
| Diabetes | 469 (39.9) | 345 (40.3) | 124 (38.9) | 0.705 |
| Malignancy | 90 (7.7) | 52 (6.1) | 38 (11.9) | 0.001 |
| Dementia | 61 (5.2) | 38 (4.4) | 23 (7.2) | 0.079 |
| AIDS | 3 (0.3) | 1 (0.1) | 2 (0.6) | 0.373 |
| Infection site, n (%) | ||||
| Lung infection | 278 (23.7) | 193 (22.5) | 85 (26.6) | 0.164 |
| Gastrointestinal infection | 60 (5.1) | 41 (4.8) | 19 (6.0) | 0.510 |
| Genito urinary infection | 209 (17.8) | 161 (18.8) | 48 (15.0) | 0.157 |
| Other | 503 (42.8) | 321 (37.5) | 182 (57.1) | < 0.001 |
| Intervention, n (%) | ||||
| Severity of illness, [M(IQR)] | ||||
| mSOFA | 4.00 (2.00, 6.00) | 4.00 (2.00, 6.00) | 6.00 (4.00, 8.00) | < 0.001 |
| Inflammatory biomarkers, [M(IQR)] | ||||
| NLR | 8.75 (4.93, 15.78) | 7.83 (4.61, 13.73) | 11.68 (6.57, 21.04) | < 0.001 |
| MLR | 0.48 (0.25, 0.87) | 0.44 (0.23, 0.78) | 0.62 (0.33, 1.13) | < 0.001 |
| PLR | 4.83 (1.37, 139.80) | 3.52 (1.18, 113.27) | 54.16 (1.94, 214.64) | < 0.001 |
Abbreviations: BMI: body mass index; CCI: Charlson Comorbidity Index; AIDS: Acquired Immunodeficiency Syndrome; SOFA: Sequential Organ Failure Assessment; mSOFA: modified Sequential Organ Failure Assessment; NLR; Neutrophil-to-Lymphocyte ratio; MLR: Monocyte-to-Lymphocyte ratio, PLR: Platelet-to-Lymphocyte ratio. Bold text indicates P < 0.05
Mortality in groups with different levels of inflammatory biomarkers
Table 2 compares mortality in groups with different levels of inflammatory biomarkers. Compared with the low levels of those inflammatory biomarkers (Q1), the high levels of inflammatory biomarkers group (Q3) of NLR, MLR, and PLR had higher mortality at 7-day, 14-day, 21-day and 28-day (P < 0.05).
Table 2.
Mortality in groups with different levels of inflammatory biomarkers
| NLR | MLR | PLR | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Q1 < 5.86 | 5.86 ≤ Q2 < 12.58 | Q3 ≥ 12.58 | P | Q1 < 0.32 | 0.32 ≤ Q2 < 0.70 | Q3 ≥ 0.70 | P | Q1 < 1.90 | 1.90 ≤ Q2 < 90.21 | Q3 ≥ 90.21 | P | |||
| Mortality, n (%) | |||||||||||||||
| 7-day | 186 (15.8) | 42 (10.7) | 52 (13.3) | 92 (23.5) | < 0.001 | 50 (12.7) | 47 (12.1) | 89 (22.7) | < 0.001 | 46 (11.8) | 57 (14.5) | 83 (21.2) | 0.001 | ||
| 14-day | 259 (22.0) | 57 (14.5) | 78 (19.9) | 124 (31.6) | < 0.001 | 66 (16.8) | 73 (18.7) | 120 (30.6) | < 0.001 | 62 (15.9) | 84 (21.4) | 113 (28.8) | < 0.001 | ||
| 21-day | 296 (25.2) | 68 (17.3) | 93 (23.8) | 135 (34.4) | < 0.001 | 78 (19.8) | 85 (21.8) | 133 (33.9) | < 0.001 | 75 (19.2) | 97 (24.7) | 124 (31.6) | < 0.001 | ||
| 28-day | 319 (27.1) | 70 (17.9) | 100 (25.6) | 149 (38.0) | < 0.001 | 79 (20.1) | 98 (25.1) | 142 (36.2) | < 0.001 | 77 (19.7) | 105 (26.8) | 137 (34.9) | < 0.001 | ||
| ICU | 233 (19.8) | 50 (12.8) | 69 (17.6) | 114 (29.1) | < 0.001 | 54 (13.7) | 68 (17.4) | 111 (28.3) | < 0.001 | 56 (14.3) | 70 (17.9) | 107 (27.3) | < 0.001 | ||
| Hospital | 271 (23.1) | 60 (15.3) | 83 (21.2) | 128 (32.7) | < 0.001 | 70 (17.8) | 73 (18.7) | 128 (32.7) | < 0.001 | 68 (17.4) | 83 (21.2) | 120 (30.6) | < 0.001 | ||
Data are presented as n (%), NLR, Neutrophil-to-Lymphocyte ratio; MLR, Monocyte-to-Lymphocyte ratio, PLR, Platelet-to-Lymphocyte ratio. Q1, low levels of inflammatory biomarkers; Q2, Medium levels of inflammatory biomarkers; Q3, high levels of inflammatory biomarkers. Bold values represent significant p values
Associations of inflammatory biomarkers with 28-day mortality in septic patients with CAD
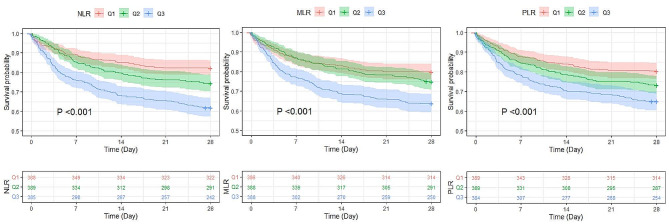
Kaplan‒Meier analysis showed that among the three inflammatory biomarkers (NLR, MLR and PLR), the group with high inflammatory biomarkers (Q3) had a higher 28-day all-cause mortality than the group with low inflammatory biomarkers (Q1) (P < 0.001) (Fig. 2). Univariate Cox proportional-hazard regression analysis showed that continuous variable NLR, PLR, and MLR were associated with 28-day mortality in septic patients with CAD. Among the three inflammatory biomarkers (NLR, MLR and PLR), the group with high inflammatory biomarkers (Q3) had a higher 28-day mortality than the group with low inflammatory biomarkers (Q1). Adjust for age, gender, BMI, race, cardiovascular disease, chronic pulmonary disease, liver disease, malignancy, dementia and mechanical ventilation, 28-day all-cause mortality of septic patients with CAD was significantly related to rising NLR levels (adjusted hazard ratio [aHR]: 1.02; 95% confidence interval [CI]: 1.01–1.02; P < 0.001), MLR levels (aHR: 1.29; 95%CI: 1.18–1.41; P < 0.001), and PLR levels (aHR: 1.0007; 95%CI: 1.0004–1.0011; P < 0.001). Meanwhile, the higher levels (Q3) group of NLR, MLR, and PLR also had a higher risk of 28-day all-cause mortality than the lower (Q1) group (Table 3).
Fig. 2.
Kaplan‒Meier analysis analysis of the difference among different levels of inflammatory biomarkers
Table 3.
Cox proportional-hazards regression analysis for associations of inflammatory biomarkers with 28-day all-cause mortality in septic patients with CAD
| Unadjusted | Model 1 | Model 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | ||
| NLR | 1.02(1.01,1.02) | < 0.001 | 1.02(1.01,1.02) | < 0.001 | 1.02(1.01,1.02) | < 0.001 | ||
| Q1 | ref | ref | ref | ref | ref | ref | ||
| Q2 | 1.47(1.08,1.99) | 0.014 | 1.43(1.05,1.95) | 0.023 | 1.42(1.04,1.93) | 0.027 | ||
| Q3 | 2.41(1.82,3.21) | < 0.001 | 2.31(1.73,3.07) | < 0.001 | 2.15(1.61,2.87) | < 0.001 | ||
| MLR | 1.30(1.19,1.41) | < 0.001 | 1.32(1.21,1.45) | < 0.001 | 1.29(1.18,1.41) | < 0.001 | ||
| Q1 | ref | ref | ref | ref | ref | ref | ||
| Q2 | 1.25(0.93,1.68) | 0.141 | 1.15(0.85,1.55) | 0.372 | 1.09(0.81,1.48) | 0.557 | ||
| Q3 | 1.98(1.51,2.61) | < 0.001 | 1.82(1.38,2.41) | < 0.001 | 1.69(1.27,2.24) | < 0.001 | ||
| PLR | 1.0008(1.0004,1.0011) | < 0.001 | 1.0007(1.0004,1.0011) | < 0.001 | 1.0007(1.0004,1.0011) | < 0.001 | ||
| Q1 | ref | ref | ref | ref | ref | ref | ||
| Q2 | 1.41(1.05,1.89) | 0.022 | 1.34(1.00,1.81) | 0.052 | 1.33(0.99,1.79) | 0.060 | ||
| Q3 | 1.96(1.48,2.59) | < 0.0001 | 1.83(1.38,2.43) | < 0.001 | 1.83(1.38,2.44) | < 0.001 | ||
HR, Hazard Ratio; CI, confidence interval; NLR, Neutrophil-to-Lymphocyte ratio; MLR, Monocyte-to-Lymphocyte ratio, PLR,
Platelet-to-Lymphocyte ratio. BMI, body mass index
Q1: low levels of inflammatory biomarkers;
Q2: Medium levels of inflammatory biomarkers;
Q3: high levels of inflammatory biomarkers
Model 1: adjust for age, gender, BMI, race
Model 2: adjust for age, gender, BMI, race, cardiovascular disease, chronic pulmonary disease, liver disease, malignancy, dementia, mechanical ventilation
Bold values represent significant p values
Predictive value of 28-day all-cause mortality in septic patients with CAD
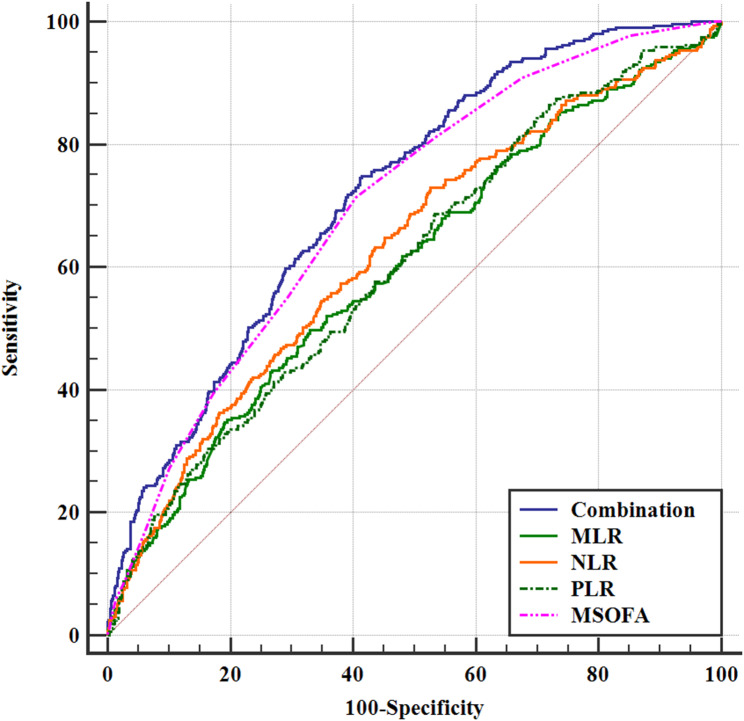
ROC analysis showed that the AUC of mSOFA scores, MLR, NLR, and PLR in predicting the 28-day mortality in septic patients with CAD was 0.699 (95%CI 0.672–0.725), 0.601 (95%CI 0.573–0.630), 0.627 (95%CI 0.599–0.655), and 0.606 (95%CI 0.577–0.634), respectively. Then the area under the curve (AUC) of the combined four indicators (mSOFA, NLR, MLR, PLR) in predicting the 28-day all-cause mortality in septic patients with CAD was 0.719 (95%CI 0.692–0.745). Thus, the combined index significantly improved the ability of predicting 28-day all-cause mortality compared to the predictive values of alone indicators (P < 0.05) (Table 4; Fig. 3).
Table 4.
ROC analysis for 28-day all-cause mortality in septic patients with CAD
| Variable | AUC | SE | 95% CI | Cut-off value | Sensitivity | Specificity | Youden index J |
|---|---|---|---|---|---|---|---|
| Combination (MLR + NLR + PLR + MSOFA) | 0.719 | 0.0158 | 0.692 to 0.745 | > 0.233 | 74.92 | 58.53 | 0.335 |
| MLR | 0.601 | 0.0189 | 0.573 to 0.630 | > 0.643 | 49.84 | 66.94 | 0.168 |
| NLR | 0.627 | 0.0186 | 0.599 to 0.655 | > 7.301 | 73.04 | 47.31 | 0.204 |
| PLR | 0.606 | 0.0185 | 0.577 to 0.634 | > 2.785 | 68.65 | 46.61 | 0.153 |
| MSOFA | 0.699 | 0.0163 | 0.672 to 0.725 | > 4 | 71.47 | 59.46 | 0.309 |
Abbreviations NLR, Neutrophil-to-Lymphocyte ratio. MLR, Monocyte-to-Lymphocyte ratio. PLR, Platelet-to-Lymphocyte ratio. mSOFA, modified Sequential Organ Failure Assessment. AUC, Area Under the Curve. CI, Confidence Interval. SE, Standard Error
Fig. 3.
Prediction value of 28-day mortality in septic patients with CAD
NLR: Neutrophil-to-Lymphocyte ratio; MLR: Monocyte-to-Lymphocyte ratio, PLR; Platelet-to-Lymphocyte ratio; mSOFA; modified sequential organ failure assessment
Discussion
Our study demonstrated that high levels of inflammatory biomarkers (NLR, MLR, and PLR) were associated with 28-day all-cause mortality in septic patients with CAD. In addition, we found that using a combination of mSOFA scores with inflammatory biomarkers levels significantly improved the ability in predicting the 28-day all-cause mortality in septic patients with CAD. Our findings had important clinical significance because the ability to predict the 28-day risk of death in septic patients with CAD using easily accessible inflammatory biomarkers and a simple scoring system will allow clinicians to improve the diagnosis and treatment times of these patients.
Although neutrophil, monocyte and lymphocyte play a crucial role in the body’s defense against pathogens, a patient’s white blood cell count may present false negatives or may be delayed in reflecting the progression of inflammatory disease. Recent studies have demonstrated that white blood cell count ratios such as NLR, MLR, and PLR may act as potential inflammation biomarkers in a variety of diseases. For example, Shi et al. found that NLR levels on day 5 in hospitalised patients were independently associated with mortality in septic patients [33], while Shen et al. reported that a high level of PLR on admission was associated with mortality in septic patients [34]. Furthermore, NLR has been shown to independently predict cardiac mortality in patients with stable CAD; a dose-response model of NLR demonstrated that the risk of death increased when the NLR was higher than 2.42 [35]. Chen et al. found that a high level of NLR was associated with myocardial dysfunction [36]. Similarly, Bressi et al. showed that elevated NLR, but not PLR, before surgery was an independent predictor of major adverse cardiac events in CAD patients [37]. Our study further extended the role of inflammatory biomarkers to septic patients with CAD. We found that non-survival group had higher NLR, MLR, and PLR values compared to the survival group, and that high NLR and PLR levels were independent risk factors for 28-day mortality in septic patients with CAD. Our findings suggested that inflammatory biomarkers were also associated with poor prognosis in the disease comorbidities, and that changes in inflammatory biomarkers reflected septic inflammation and progression of myocardial damage.
We further explored the ability of inflammatory biomarkers in predicting 28-day all-casue mortality in septic patients with CAD. We found that NLR, MLR, and PLR predicted 28-day mortality in septic patients with CAD. The AUCs of NLR, MLR, and PLR were 0.627, 0.601, and 0.606, respectively. Similarly, Liu et al. reported that the AUC of predicting 28-day mortality in septic patients by NLR was 0.634, and its sensitivity and specificity were 78.3% and 50%, respectively [38]. Shi et al. found that the AUC of predicting mortality in septic patients using NLR on day 5 of admission was 0.589, and the sensitivity and specificity were 32.3% and 83.3% [33], respectively. Together, these findings suggested that the inflammatory biomarkers were similar in predicting mortality. In addition, these studies also suggested that single inflammatory biomarkers were insufficient to predict disease mortality accurately. Thus, in order to improve the predictive value of mortality in such patients, a better combination of indicators may be required.
SOFA scores were an important part of sepsis-3 criteria [27]. Previous studies have reported that SOFA scores were a useful early prognostic marker for predicting 28-day mortality in septic patients [39]. However, in recent years, there is a more critical attitude towards the SOFA score, as many studies have identified the limitations of the original version of the SOFA score in various accompanying diseases (COPD, chronic kidney disease, malignancies, etc.). At the same time, modifications of the SOFA score regarding the presence of individual organ system failure (mSOFA) have been developed in recent years. Due to the reduced importance of the original version of the SOFA score, especially in the case of cardio-vascular failure, and the unreliable criterion for CAD, the final results are also less reliable [24, 40]. Therefore, in the present study, we combined mSOFA scores rather than SOFA socre with inflammatory biomarkers values to predict 28-day all-cause mortality of septic patients with CAD. Our study found that although the ability of mSOFA scores in predicting the 28-day all-cause mortality of septic patients with CAD was insufficient, using a combination of inflammatory biomarkers with mSOFA scores could improve the ability of predicting 28-day mortality in patients with sepsis and CAD. These findings have important clinical significance, the diagnosis and treatment times of septic patients with CAD can be improved and 28-day all cause mortality of these patients can be predicted by using a combination of these easily accessible inflammatory biomarkers and a simple scoring system.
There were some limitations in this study. First, it was imperative to acknowledge that the MIMIC-IV database collects data over a time span of more than a decade. The ICD-9 did not have classification for defining sepsis, so previous studies identified septic patients in the MIMIC database by using codes. Due to changes in the definition of sepsis, patients identified as sepsis according to the sepsis 3.0 definition may include individuals who were not originally diagnosed with sepsis, potentially impacting treatment decisions. However, due to temporal drift in the MIMIC database, we were unable to extract septic patients based on different periods of sepsis definitions in the database. Our findings might be influenced by changes in the sepsis guidelines that occurred during this period, which could affect the practical relevance of our results. Secondly, CAD was defined according to the codes 410–411 (ICD-9) and I20-I21 (ICD-10) from disease history and medical record diagnosis, including previous myocardial infarction, or acute coronary syndrome etc. It was unclear whether these patients with CAD underwent coronary angiography and belong to obstructive coronary artery disease. Future prospective studies are needed to verify our conclusions. Thirdly, it is necessary to identify the source of the infection and the likely bacteria causing the sepsis, we cannot confirm the associations between different bacteria and different inflammatory profiles that affect NLR in particular due to no available additional data [41–43].
Conclusions
Our study confirmed that high NLR, MLR, and PLR were associated with 28-day mortality in septic patients with CAD. Our results also demonstrated that using a combination of mSOFA scores and inflammatory biomarkers values significantly improve the diagnostic efficiency in predicting the 28-day mortality in septic patients with CAD. In the future, we need to combine laboratory tests, clinical symptoms, and scores to construct a simple, timely and efficient predictive model for the short-term risk of mortality in septic patients with CAD, in order to increase early intervention and decrease the risk of poor prognosis of these patients.
Acknowledgements
Not applicable.
Author contributions
LLF, GRW, LXC, CYB conceived and designed the study. LLF, GRW, LXC, CYB, YQ and ZHY conducted the research. CYB and LLF analyzed the data. LLF and LXC wrote the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by the Science and Technology Department of Yunnan Province (202101AY070001-030) and the 920th Hospital of Joint Logistics Support Force, PLA (2020YGD11).
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethical approval and consent to participate
To safeguard patient privacy, all personally identifiable information has been anonymized. As all patient records in the MIMIC-IV database were thoroughly de-identified, the need for individual patient consent was deemed unnecessary by the institutional review board of the Beth Israel Deaconess Medical Center, and the study was conducted out in conformity with the Declaration of Helsinki. After going through a “CITI Data or Specimens Only Research” training course on the National Institutes of Health (NIH) website, the author is approved to extract data from this database for research purposes (Certificate No: 40416369).
Consent for publication
Not applicable.
Competing interests
The authors of this study have no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xicong Li and Yubiao Chen contributed equally to this work.
Contributor Information
Lifei Lu, Email: lulifeiwork@163.com.
Ruiwei Guo, Email: grw771210@163.com.
References
- 1.Machado FR, Cavalcanti AB, Bozza FA, Ferreira EM, Angotti Carrara FS, Sousa JL, Caixeta N, Salomao R, Angus DC, Pontes Azevedo LC. The epidemiology of sepsis in Brazilian intensive care units (the Sepsis PREvalence Assessment Database, SPREAD): an observational study. Lancet Infect Dis. 2017;17:1180–9. 10.1016/S1473-3099(17)30322-5 [DOI] [PubMed] [Google Scholar]
- 2.Incidence of severe sepsis. And septic shock in German intensive care units: the prospective, multicentre INSEP study. Intensive Care Med. 2016;42:1980–9. 10.1007/s00134-016-4504-3 [DOI] [PubMed] [Google Scholar]
- 3.Kern H, Wittich R, Rohr U, Kox WJ, Spies CD. Increased endothelial injury in septic patients with coronary artery disease. Chest. 2001;119:874–83. 10.1378/chest.119.3.874 [DOI] [PubMed] [Google Scholar]
- 4.Dolmatova EV, Wang K, Mandavilli R, Griendling KK. The effects of sepsis on endothelium and clinical implications. Cardiovasc Res. 2021;117:60–73. 10.1093/cvr/cvaa070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lv X, Wang H. Pathophysiology of sepsis-induced myocardial dysfunction. Mil Med Res. 2016;3:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smilowitz NR, Gupta N, Guo Y, Bangalore S. Comparison of outcomes of patients with Sepsis with Versus without Acute myocardial infarction and comparison of Invasive Versus Noninvasive Management of the patients with infarction. Am J Cardiol. 2016;117:1065–71. 10.1016/j.amjcard.2015.12.050 [DOI] [PubMed] [Google Scholar]
- 7.Merx MW, Weber C. Sepsis and the heart. Circulation. 2007;116:793–802. 10.1161/CIRCULATIONAHA.106.678359 [DOI] [PubMed] [Google Scholar]
- 8.Arbel Y, Finkelstein A, Halkin A, Birati EY, Revivo M, Zuzut M, Shevach A, Berliner S, Herz I, Keren G, Banai S. Neutrophil/lymphocyte ratio is related to the severity of coronary artery disease and clinical outcome in patients undergoing angiography. Atherosclerosis. 2012;225:456–60. 10.1016/j.atherosclerosis.2012.09.009 [DOI] [PubMed] [Google Scholar]
- 9.Wheeler JG, Mussolino ME, Gillum RF, Danesh J. Associations between differential leucocyte count and incident coronary heart disease: 1764 incident cases from seven prospective studies of 30,374 individuals. Eur Heart J. 2004;25:1287–92. 10.1016/j.ehj.2004.05.002 [DOI] [PubMed] [Google Scholar]
- 10.Gao Y, Li Y, Chen X, Wu C, Guo Z, Bai G, Liu T, Li G. The systemic inflammation index predicts poor clinical prognosis in patients with initially diagnosed Acute Coronary Syndrome undergoing primary coronary angiography. J Inflamm Res. 2023;16:5205–19. 10.2147/JIR.S435398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tudurachi BS, Anghel L, Tudurachi A, Sascău RA, Stătescu C. Assessment of Inflammatory Hematological Ratios (NLR, PLR, MLR, LMR and Monocyte/HDL-Cholesterol ratio) in Acute Myocardial Infarction and particularities in Young patients. Int J Mol Sci 2023, 24. [DOI] [PMC free article] [PubMed]
- 12.Shi S, Kong S, Ni W, Lu Y, Li J, Huang Y, Chen J, Lin K, Li Y, Ke J, Zhou H. Association of the systemic Immune-inflammation index with outcomes in Acute Coronary Syndrome patients with chronic kidney disease. J Inflamm Res. 2023;16:1343–56. 10.2147/JIR.S397615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang X, Wei R, Wang X, Zhang W, Li M, Ni T, Weng W, Li Q. The neutrophil-to-lymphocyte ratio is associated with all-cause and cardiovascular mortality among individuals with hypertension. Cardiovasc Diabetol. 2024;23:117. 10.1186/s12933-024-02191-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hong S, He H, Fang P, Liu S, Chen C. Association of neutrophil-to-lymphocyte ratio and risk of cardiovascular and all-cause mortality in hypertension patients. Heliyon. 2024;10:e27517. 10.1016/j.heliyon.2024.e27517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong G, Gan M, Xu S, Xie Y, Zhou M, Wu L. The neutrophil-lymphocyte ratio as a risk factor for all-cause and cardiovascular mortality among individuals with diabetes: evidence from the NHANES 2003–2016. Cardiovasc Diabetol. 2023;22:267. 10.1186/s12933-023-01998-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Djordjevic D, Rondovic G, Surbatovic M, Stanojevic I, Udovicic I, Andjelic T, Zeba S, Milosavljevic S, Stankovic N, Abazovic D et al. Neutrophil-to-Lymphocyte Ratio, Monocyte-to-Lymphocyte Ratio, Platelet-to-Lymphocyte Ratio, and Mean Platelet Volume-to-Platelet Count Ratio as Biomarkers in Critically Ill and Injured Patients: Which Ratio to Choose to Predict Outcome and Nature of Bacteremia? Mediators Inflamm 2018, 2018:3758068. [DOI] [PMC free article] [PubMed]
- 17.Spoto S, Lupoi DM, Valeriani E, Fogolari M, Locorriere L, Beretta Anguissola G, Battifoglia G, Caputo D, Coppola A, Costantino S et al. Diagnostic accuracy and Prognostic Value of Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios in septic patients outside the Intensive Care Unit. Med (Kaunas) 2021, 57. [DOI] [PMC free article] [PubMed]
- 18.Huang Z, Fu Z, Huang W, Huang K. Prognostic value of neutrophil-to-lymphocyte ratio in sepsis: a meta-analysis. Am J Emerg Med. 2020;38:641–7. 10.1016/j.ajem.2019.10.023 [DOI] [PubMed] [Google Scholar]
- 19.Wu H, Cao T, Ji T, Luo Y, Huang J, Ma K. Predictive value of the neutrophil-to-lymphocyte ratio in the prognosis and risk of death for adult sepsis patients: a meta-analysis. Front Immunol. 2024;15:1336456. 10.3389/fimmu.2024.1336456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Russell CD, Parajuli A, Gale HJ, Bulteel NS, Schuetz P, de Jager CPC, Loonen AJM, Merekoulias GI, Baillie JK. The utility of peripheral blood leucocyte ratios as biomarkers in infectious diseases: a systematic review and meta-analysis. J Infect. 2019;78:339–48. 10.1016/j.jinf.2019.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sasmita BR, Zhu Y, Gan H, Hu X, Xue Y, Xiang Z, Huang B, Luo S. Prognostic value of neutrophil-lymphocyte ratio in cardiogenic shock complicating acute myocardial infarction: a cohort study. Int J Clin Pract. 2021;75:e14655. 10.1111/ijcp.14655 [DOI] [PubMed] [Google Scholar]
- 22.Gazi E, Bayram B, Gazi S, Temiz A, Kirilmaz B, Altun B, Barutcu A. Prognostic value of the neutrophil-lymphocyte ratio in patients with ST-Elevated acute myocardial infarction. Clin Appl Thromb Hemost. 2015;21:155–9. 10.1177/1076029613492011 [DOI] [PubMed] [Google Scholar]
- 23.Sia CH, Leow AS, Tan BY, Low CJ, Kaur R, Yeo TC, Chan MY, Tay EL, Yeo LL, Yap ES, Loh JP. The neutrophil-lymphocyte ratio and platelet-lymphocyte ratio predict left ventricular thrombus resolution in acute myocardial infarction without percutaneous coronary intervention. Thromb Res. 2020;194:16–20. 10.1016/j.thromres.2020.06.003 [DOI] [PubMed] [Google Scholar]
- 24.Wang X, Guo Z, Chai Y, Wang Z, Liao H, Wang Z, Wang Z. Application Prospect of the SOFA score and Related Modification Research Progress in Sepsis. J Clin Med 2023, 12. [DOI] [PMC free article] [PubMed]
- 25.Johnson AEW, Bulgarelli L, Shen L, Gayles A, Shammout A, Horng S, Pollard TJ, Hao S, Moody B, Gow B, et al. MIMIC-IV, a freely accessible electronic health record dataset. Sci Data. 2023;10:1. 10.1038/s41597-022-01899-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu W, Chen H, Ma C, Sun Q, Yang M, Wang H, Peng Q, Wang J, Zhang C, Huang W, et al. Identification of indications for albumin administration in septic patients with liver cirrhosis. Crit Care. 2023;27:300. 10.1186/s13054-023-04587-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, et al. The Third International Consensus definitions for Sepsis and septic shock (Sepsis-3). JAMA. 2016;315:801–10. 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson AEW, Aboab J, Raffa JD, Pollard TJ, Deliberato RO, Celi LA, Stone DJ. A comparative analysis of Sepsis Identification methods in an electronic database. Crit Care Med. 2018;46:494–9. 10.1097/CCM.0000000000002965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ye Z, An S, Gao Y, Xie E, Zhao X, Guo Z, Li Y, Shen N, Zheng J. Association between the triglyceride glucose index and in-hospital and 1-year mortality in patients with chronic kidney disease and coronary artery disease in the intensive care unit. Cardiovasc Diabetol. 2023;22:110. 10.1186/s12933-023-01843-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He HM, Xie YY, Wang Z, Li J, Zheng SW, Li XX, Jiao SQ, Yang FR, Sun YH. Associations of variability in blood glucose and systolic blood pressure with mortality in patients with coronary artery disease: a retrospective cohort study from the MIMIC-IV database. Diabetes Res Clin Pract. 2024;209:111595. 10.1016/j.diabres.2024.111595 [DOI] [PubMed] [Google Scholar]
- 31.He HM, Zheng SW, Xie YY, Wang Z, Jiao SQ, Yang FR, Li XX, Li J, Sun YH. Simultaneous assessment of stress hyperglycemia ratio and glycemic variability to predict mortality in patients with coronary artery disease: a retrospective cohort study from the MIMIC-IV database. Cardiovasc Diabetol. 2024;23:61. 10.1186/s12933-024-02146-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luo J, Zhou Y, Song Y, Wang D, Li M, Du X, Kang J, Ye P, Xia J. Association between the neutrophil-to-lymphocyte ratio and in-hospital mortality in patients with chronic kidney disease and coronary artery disease in the intensive care unit. Eur J Med Res. 2024;29:260. 10.1186/s40001-024-01850-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi Y, Yang C, Chen L, Cheng M, Xie W. Predictive value of neutrophil-to-lymphocyte and platelet ratio in in-hospital mortality in septic patients. Heliyon. 2022;8:e11498. 10.1016/j.heliyon.2022.e11498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shen Y, Huang X, Zhang W. Platelet-to-lymphocyte ratio as a prognostic predictor of mortality for sepsis: interaction effect with disease severity-a retrospective study. BMJ Open. 2019;9:e022896. 10.1136/bmjopen-2018-022896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Papa A, Emdin M, Passino C, Michelassi C, Battaglia D, Cocci F. Predictive value of elevated neutrophil-lymphocyte ratio on cardiac mortality in patients with stable coronary artery disease. Clin Chim Acta. 2008;395:27–31. 10.1016/j.cca.2008.04.019 [DOI] [PubMed] [Google Scholar]
- 36.Chen C, Cong BL, Wang M, Abdullah M, Wang XL, Zhang YH, Xu SJ, Cui L. Neutrophil to lymphocyte ratio as a predictor of myocardial damage and cardiac dysfunction in acute coronary syndrome patients. Integr Med Res. 2018;7:192–9. 10.1016/j.imr.2018.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bressi E, Mangiacapra F, Ricottini E, Cavallari I, Colaiori I, Di Gioia G, Creta A, Capuano M, Viscusi MM, Di Sciascio G. Impact of Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio on 5-Year clinical outcomes of patients with stable coronary artery Disease Undergoing Elective Percutaneous Coronary intervention. J Cardiovasc Transl Res. 2018;11:517–23. 10.1007/s12265-018-9829-6 [DOI] [PubMed] [Google Scholar]
- 38.Liu Y, Zheng J, Zhang D, Jing L. Neutrophil-lymphocyte ratio and plasma lactate predict 28-day mortality in patients with sepsis. J Clin Lab Anal. 2019;33:e22942. 10.1002/jcla.22942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karakike E, Kyriazopoulou E, Tsangaris I, Routsi C, Vincent JL, Giamarellos-Bourboulis EJ. The early change of SOFA score as a prognostic marker of 28-day sepsis mortality: analysis through a derivation and a validation cohort. Crit Care. 2019;23:387. 10.1186/s13054-019-2665-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martín-Rodríguez F, Sanz-García A, Del Pozo Vegas C, Ortega GJ, Castro Villamor MA, López-Izquierdo R. Time for a prehospital-modified sequential organ failure assessment score: an ambulance-based cohort study. Am J Emerg Med. 2021;49:331–7. 10.1016/j.ajem.2021.06.042 [DOI] [PubMed] [Google Scholar]
- 41.Chen Y, Ye LJ, Wu Y, Shen BZ, Zhang F, Qu Q, Qu J. Neutrophil-Lymphocyte Ratio in Predicting Infective Endocarditis: A Case-Control Retrospective Study. Mediators Inflamm 2020, 2020:8586418. [DOI] [PMC free article] [PubMed]
- 42.Kim MA, Park YE, Chong YP, Shim TS, Jo KW. Neutrophil-lymphocyte ratio and monocyte-lymphocyte ratio according to the Radiologic Severity of Mycobacterium avium Complex Pulmonary Disease. J Korean Med Sci. 2022;37:e292. 10.3346/jkms.2022.37.e292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seyedi SA, Nabipoorashrafi SA, Hernandez J, Nguyen A, Lucke-Wold B, Nourigheimasi S, Khanzadeh S. Neutrophil to Lymphocyte Ratio and Spontaneous Bacterial Peritonitis among Cirrhotic Patients: A Systematic Review and Meta-analysis. Can J Gastroenterol Hepatol 2022, 2022:8604060. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.