Abstract
Objective
First-line bevacizumab plus carboplatin and paclitaxel (CP) is approved for stage III/IV ovarian cancer treatment following initial surgical resection, based on global phase III GOG-0218 and ICON7 trials. This study evaluated the efficacy and safety of bevacizumab + CP as first-line ovarian cancer therapy in Chinese patients.
Methods
Patients with newly diagnosed, International Federation of Gynecology and Obstetrics (FIGO) stage III/IV epithelial ovarian, fallopian tube, or primary peritoneal cancer post-primary surgery were randomized 1:1 to receive 6 cycles of CP with bevacizumab/placebo, followed by bevacizumab/placebo maintenance until unacceptable toxicity or disease progression. Primary endpoint was investigator-assessed progression-free survival (PFS). Stratification factors were FIGO stage and debulking status (stage III optimally debulked vs stage III suboptimally debulked vs stage IV) and Eastern Cooperative Oncology Group performance status (0 vs 1 or 2).
Results
Of randomized patients, 51 received bevacizumab + CP and 49 received placebo + CP. Median PFS was 22.6 months with bevacizumab + CP (95% confidence interval [CI]=18.6, not estimable) and 12.3 months (95% CI=9.5, 15.0) with placebo + CP (stratified hazard ratio=0.30; 95% CI=0.17, 0.53). Treatment-related grade 3/4 adverse events occurred in 46 of 49 (94%) patients receiving bevacizumab + CP, and 34 of 50 (68%) receiving placebo + CP.
Conclusion
Bevacizumab + CP showed clinically meaningful improvement in PFS vs placebo + CP, consistent with GOG-0218 results. Safety data were aligned with the known bevacizumab safety profile. These results support first-line bevacizumab + CP therapy in Chinese patients with ovarian cancer.
Trial Registration
ClinicalTrials.gov Identifier: NCT03635489
Keywords: Bevacizumab, Chemotherapy, Chinese, Ovarian Cancer
Synopsis
First-line bevacizumab + carboplatin and paclitaxel (CP) showed clinically meaningful improvement in progression-free survival vs placebo + CP and was well tolerated in patients with ovarian cancer. Results of this phase III randomized controlled study are consistent with global data and support the use of bevacizumab + CP in Chinese patients.
INTRODUCTION
Epithelial ovarian cancer, fallopian tube cancer, and primary peritoneal cancer (hereafter referred to as ovarian cancer) remain the leading causes of cancer-related mortality among women globally. In 2020, 313,959 new cases and 207,252 deaths were attributed to ovarian cancer [1], with China accounting for 55,342 (18%) of these new cases and 37,519 (18%) of deaths [2]. Due to its characteristically asymptomatic nature, diagnosis of ovarian cancer remains uncommon at an early stage [3]; hence, most patients are with disseminated and advanced-stage disease at initial diagnosis. The 5-year survival rate for patients with stage I ovarian cancer is 89%, compared with 41% and 20% for patients with advanced stage III and IV ovarian cancer, respectively [4].
For decades, the standard of care for first-line treatment of ovarian cancer has been primary tumor reduction surgery, followed by carboplatin plus paclitaxel (CP) chemotherapy [5]. Although ovarian cancer is highly sensitive to initial CP treatment, 50%–75% of responders relapse within approximately 18 months, and eventually succumb to the cancer [6,7]. Clinical experience shows that adding a biologic agent, such as bevacizumab, to CP can improve survival rates [8].
Bevacizumab is a recombinant, humanized, monoclonal antibody that binds to the vascular endothelial growth factor and neutralizes its activity, inhibiting angiogenesis [9]. Two randomized phase III studies, GOG-0218 and ICON7, demonstrated the efficacy and safety of bevacizumab + CP in treatment-naive patients with advanced ovarian cancer [8,10,11,12]. These results supported the US Food and Drug Administration’s (FDA) approval of first-line bevacizumab + CP followed by single-agent maintenance with bevacizumab, for the treatment of stage III/IV ovarian cancer following initial surgical resection [13]. Several real-world studies have also confirmed the safety and efficacy of first-line bevacizumab + CP in patients with ovarian cancer [14,15,16]. The efficacy of bevacizumab + CP was further demonstrated in the first-line treatment of ovarian cancer in high-risk patients (older, less fit, and had more comorbidities than typical phase III clinical trial populations; OTILIA study) [17], and in the second-line treatment of patients with platinum-sensitive ovarian cancer (phase III MITO16b/MANGO–OV2/ENGOT–ov17 trial) [18].
Recent significant advancements in ovarian cancer treatment modalities include the FDA approval of poly (ADP-ribose) polymerase (PARP) inhibitors, such as olaparib, rucaparib and niraparib, for the first-line maintenance treatment of patients with advanced ovarian cancer and in the recurrent setting [19,20,21,22]. In China, olaparib and niraparib are approved as first-line maintenance treatment for advanced ovarian cancer in select patient populations [23,24], and fluzoparib is approved for the treatment of recurrent ovarian cancer [25] by the National Medical Products Administration. Despite these developments, primary therapy with bevacizumab + CP followed by bevacizumab maintenance remains a widely used and preferred regimen for ovarian cancer recommended by international guidelines [26].
Although Chinese patients represent almost one-fifth of global ovarian cancer cases, data on the efficacy of bevacizumab in this population are currently lacking. Both GOG-0218 and ICON7, as well as real-world studies on bevacizumab, were conducted in predominantly Caucasian populations and did not include Chinese patients with ovarian cancer [8,10,11,12]. Thus, this phase III, placebo-controlled, randomized study (YO40268; ClinicalTrials.gov: NCT03635489), evaluated the efficacy and safety of bevacizumab + CP as first-line therapy in Chinese patients with ovarian cancer. The objectives of YO40268 were to confirm the efficacy and safety of bevacizumab in this population of Chinese women, and to investigate the consistency in treatment outcomes between Chinese patients and patients in the global GOG-0218 study.
MATERIALS AND METHODS
1. Patients
Patients with newly diagnosed, previously untreated, International Federation of Gynecology and Obstetrics (FIGO) stage III with any gross (macroscopic or palpable) residual disease, or FIGO stage IV (regardless of residual disease) epithelial ovarian, fallopian tube, or primary peritoneal cancer were enrolled in this study. Key inclusion criteria included age ≥18 years, Eastern Cooperative Oncology Group (ECOG) performance status 0, 1, or 2, and adequate hematological, renal, and liver function. Patients were excluded if they had borderline epithelial ovarian tumors, recurrent ovarian cancer treated with surgery alone, or a history of stage IB or above endometrial cancer. Key inclusion and exclusion criteria are provided in the Data S1. YO40268 was conducted in accordance with the International Conference on Harmonization guidelines for Good Clinical Practice and the principles of the Declaration of Helsinki. Protocol approval was obtained from the Institutional Review Board or ethics committee at each site. All patients provided written informed consent.
2. Study design
This phase III, double-blind, placebo-controlled, randomized study (NCT03635489) was designed to confirm the efficacy and safety of bevacizumab in a population of Chinese women with advanced ovarian, fallopian tube, and primary peritoneal cancers who have not received prior chemotherapy for this disease. This study further aimed to investigate the consistency in treatment outcomes between Chinese patients and patients in the pivotal global GOG-0218 study and fill the evidence gap regarding the efficacy and safety of bevacizumab in Chinese patients. The primary efficacy results were bridged to those of the pivotal study GOG218. Eligible patients were randomized 1:1 to receive either bevacizumab + CP, or placebo + CP. Further details on randomization and sample size determination are provided in the Data S1.
All eligible patients received a total of 6 cycles of carboplatin (area under the curve) 6 mg/mL/min plus paclitaxel 175 mg/m2 administered once every 3 weeks, with either bevacizumab 15 mg/kg or placebo (Fig. S1). Bevacizumab or placebo was initiated at cycle 2; after completing the 6 cycles of CP, bevacizumab (15 mg/kg, once every 3 weeks) or placebo was given as a single agent for up to 16 cycles until occurrence of unacceptable toxicity or disease progression, as defined by the Response Evaluation Criteria in Solid Tumours (RECIST) [27].
The primary efficacy endpoint was investigator-assessed progression-free survival (PFS) per RECIST v1.1, defined as time from randomization to first occurrence of disease progression, or death from any cause during the study, whichever occurred first. Per RECIST v1.1, disease progression was defined as a ≥20% relative increase (from the smallest sum on study, including baseline), and an absolute increase ≥5 mm in the sum of diameters of target lesions. The appearance of ≥1 new lesion was also considered progression. Secondary endpoints included overall survival (OS; defined as time from randomization to death from any cause) in the intention-to-treat population; objective response rate (defined as confirmed complete or partial response according to RECIST v1.1 in patients with measurable residual disease after primary surgery); duration of response in patients who had an objective response; patient-reported outcomes (Data S1); and the occurrence and severity of adverse events. Severity of adverse events was determined according to National Cancer Institute Common Terminology Criteria for Adverse Events v4.0 and change from baseline in targeted vital signs and in targeted clinical laboratory test results.
3. Statistical analysis
Kaplan–Meier methodology was used to estimate median PFS, and Brookmeyer–Crowley methodology was used to construct the 95% confidence interval (CI) for the median. Hazard ratios (HRs) were estimated by Cox regression and stratified by FIGO stage and debulking status (stage III optimally debulked vs stage III suboptimally debulked vs stage IV) per electronic case report forms. Patients with stage III disease were defined as having optimally debulked tumors, if the largest maximal diameter of any residual tumor implant at the completion of initial surgery was ≤1 cm.
For subgroup analysis, unstratified HR was relative to placebo and 95% CIs were estimated by Cox regression. OS and duration of response analyses were conducted using unstratified Kaplan–Meier and Brookmeyer–Crowley methodologies. The 95% CIs for objective response rates were constructed using the Clopper–Pearson method.
Due to its bridging study design, YO40268 was not powered to demonstrate statistical significance of treatment benefit on the primary and secondary efficacy endpoints, and no formal hypothesis testing was performed.
RESULTS
1. Patients
Between August 15, 2018 and December 25, 2019, 100 patients were recruited from 16 centers in mainland China, with 51 patients randomized to the bevacizumab + CP arm and 49 patients to the placebo + CP arm (Fig. S2).
In the intention-to-treat population, patient demographics and baseline characteristics were generally balanced across both treatment arms (Table 1). Median age of patients was 53 years and most had ECOG performance status 1 or 2 (n=82; 82%) and abnormal cancer antigen-125 (n=93; 93%). Most patients were classified as stage III optimally debulked (n=48; 48%), with the ovary as the primary cancer site (n=93; 93%). Measurable disease at baseline was seen in a higher percentage of patients receiving placebo + CP (57%, n=28) than bevacizumab + CP (47%, n=24).
Table 1. Baseline demographics and disease characteristics of Chinese patients by treatment group in the ITT population.
| Characteristics | Bev + CP (n=51) | Placebo + CP (n=49) | All patients (n=100) | |
|---|---|---|---|---|
| Age (yr) | 53 (33–70) | 52 (34–75) | 53 (33–75) | |
| Age ≥65 yr | 6 (12) | 6 (12) | 12 (12) | |
| Ethnicity | ||||
| Not Hispanic or Latino | 51 (100) | 49 (100) | 100 (100) | |
| Race | ||||
| Asian* | 51 (100) | 49 (100) | 100 (100) | |
| ECOG PS† | ||||
| 0 | 10 (20) | 8 (16) | 18 (18) | |
| 1/2 | 41 (80) | 41 (84) | 82 (82) | |
| CA125 abnormal | 46 (90) | 47 (96) | 93 (93) | |
| FIGO stage and debulking status at baseline† | ||||
| Stage III optimally debulked | 25 (49) | 23 (47) | 48 (48) | |
| Stage III suboptimally debulked | 18 (35) | 18 (37) | 36 (36) | |
| Stage IV | 8 (16) | 8 (16) | 16 (16) | |
| Site of primary tumor | ||||
| Fallopian tube | 2 (4) | 2 (4) | 4 (4) | |
| Ovary | 49 (96) | 44 (90) | 93 (93) | |
| Peritoneum | 0 | 3 (6) | 3 (3) | |
| Measurable disease | 24 (47) | 28 (57) | 52 (52) | |
| Histological type | ||||
| High-grade serous | 44 (86) | 40 (82) | 84 (84) | |
| Low-grade serous | 4 (8) | 4 (8) | 8 (8) | |
| Clear cell | 1 (2) | 2 (4) | 3 (3) | |
| Mucinous | 0 | 2 (4) | 2 (2) | |
| Mixed | 2 (4) | 1 (2) | 3 (3) | |
Values are presented as median (range) or number (%).
Bev, bevacizumab; CA, cancer antigen; CP, carboplatin plus paclitaxel; ECOG PS, Eastern Cooperative Oncology Group performance status; FIGO, International Federation of Gynecology and Obstetrics; ITT, intention-to-treat.
*All patients were Chinese; †Per electronic case report form, not interactive voice/web response system.
2. Efficacy
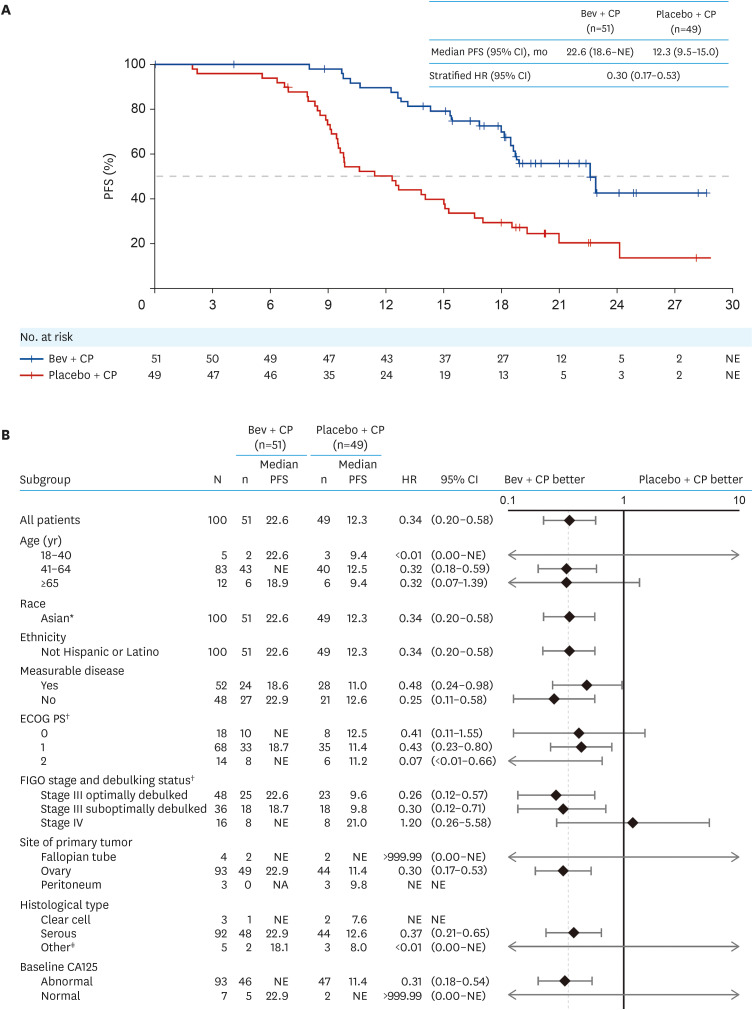
At clinical cutoff (May 28, 2021), median follow-up duration was 20.9 months in the bevacizumab + CP arm (range=0.0–28.6), and 21.0 months in the placebo + CP arm (range=2.5–33.2). Median PFS was 22.6 months in the bevacizumab+ CP arm (95% CI=18.6, not estimable), and 12.3 months (95% CI=9.5, 15.0) in the placebo + CP arm (stratified HR=0.30; 95% CI=0.17, 0.53; Fig. 1A). A higher percentage of patients experienced disease progression or death in the placebo + CP (77.6%, n=38) than the bevacizumab + CP (41.2%, n=21) arm. The percentage of patients who did not experience disease progression or death at 12 months (12-month PFS rate) in the bevacizumab + CP arm was higher than that in the placebo + CP arm (89.6% vs 50.1%). Unstratified PFS analysis of predefined subgroups based on demographics, baseline characteristics and ovarian cancer history yielded consistent results with that of the intention-to-treat population (Fig. 1B).
Fig. 1. PFS per investigator-assessed RECIST v1.1 in the ITT population. (A) Kaplan–Meier curves and (B) forest plots for primary and subgroup analyses.
Bev, bevacizumab; CA, cancer antigen; CI, confidence interval; CP, carboplatin plus paclitaxel; ECOG PS, Eastern Cooperative Oncology Group performance status; FIGO, International Federation of Gynecology and Obstetrics; HR, hazard ratio; ITT, intention-to-treat; NE, not estimable; PFS, progression-free survival; RECIST, Response Evaluation Criteria in Solid Tumours.
*All patients were Chinese.
†Per electronic case report form, not interactive voice/web response system.
‡Includes those with mixed and mucinous histology.
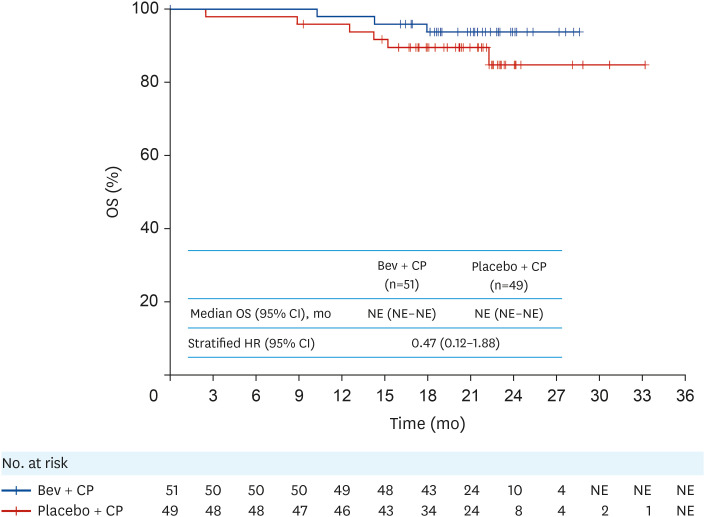
OS data were immature at clinical cutoff, with numerically lower number of deaths in the bevacizumab + CP arm (5.9%, n=3) compared with the placebo + CP arm (12.2%, n=6) in the intention-to-treat population (unstratified HR=0.47; 95% CI=0.12, 1.88; Fig. 2). Objective response rate (unconfirmed) per investigator-assessed RECIST v1.1 was 96% in patients receiving bevacizumab + CP and 93% in those receiving placebo + CP, with a difference of 3% (95% CI=−13, 19; Table 2). Complete response was achieved in 4 patients (17%) receiving bevacizumab + CP and in 4 (14%) receiving placebo + CP. Median duration of response was 16.1 months (95% CI=10.81, not estimable) and 8.9 months (95% CI=7.56, 13.14) in the bevacizumab + CP and placebo + CP arms, respectively.
Fig. 2. Kaplan–Meier curves for OS in the ITT population.
HRs were measured by unstratified Cox regression.
Bev, bevacizumab; CI, confidence interval; CP, carboplatin plus paclitaxel; HR, hazard ratio; ITT, intention-to-treat; NE, not estimable; OS, overall survival.
Table 2. Overall response rates and duration of response in the ITT population.
| Overall response | Bev + CP | Placebo + CP | |
|---|---|---|---|
| Patients with measurable disease at baseline | No. | 24 | 28 |
| ORR*,† | n (%) [95% CI] | 23 (96) [79–100] | 26 (93) [77–99] |
| CR | No. (%) | 4 (17) | 4 (14) |
| PR | No. (%) | 19 (79) | 22 (79) |
| SD | No. (%) | 1 (4) | 1 (4) |
| PD | No. (%) | 0 | 1 (4) |
| DOR (mo)†,‡ | Median (95% CI) | 16.1 (10.8, NE) | 8.9 (7.6, 13.1) |
| Unstratified HR | HR (95% CI) | 0.48 (0.24, 0.99) |
Bev, bevacizumab; CI, confidence interval; CP, carboplatin plus paclitaxel; CR, complete response, DOR, duration of response; HR, hazard ratio; ITT, intention-to-treat; NE, not evaluable; ORR, objective response rate; PD, progressive disease; PR, partial response; RECIST, Response Evaluation Criteria in Solid Tumours; SD, stable disease.
*Percentages were calculated based on the number of patients with measurable disease at baseline.
†Not confirmed response. Confirmed response was defined as a CR or PR on 2 consecutive occasions ≥4 weeks apart, as per RECIST v1.1 for patients with measurable residual disease after primary surgery.
‡DOR was assessed in patients who had an objective response (CR or PR) per investigator-assessed RECIST v1.1.
3. Patient-reported outcomes
All patients in both arms completed the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire C30 (QLQ-C30), and Quality of Life Questionnaire Ovarian Cancer Module (QLQ-OV28) at baseline. Questionnaire completion rates across patient visits during the first year of survival follow-up was 50%–80% in the bevacizumab + CP arm and 36%–59% in the placebo + CP arm. Over the treatment period, similar percentages of patients in each arm reported clinically meaningful improvement (≥10-point increase from baseline) in patient-reported function and health-related quality of life (QoL) per the EORTC QLQ-C30 global health status/QoL scales (Table S1). The percentage of patients reporting clinically meaningful improvement in both abdominal pain and bloating (per abdominal/gastrointestinal scale of the QLQ-OV28) was also comparable between both arms.
4. Safety
The safety evaluable population comprised 49 patients in the bevacizumab + CP arm and 50 patients in the placebo + CP arm. In the bevacizumab + CP arm, one patient withdrew from treatment and was excluded from the safety analysis. Additionally, 2 patients did not receive bevacizumab + CP treatment and were instead included in the safety evaluable population of the placebo + CP arm. In the placebo + CP arm, one patient received a partial dose of bevacizumab and was hence included in the safety analysis of the bevacizumab + CP arm. All patients experienced ≥1 adverse event (Table 3); no grade 5 adverse events occurred in either arm. In the bevacizumab + CP arm, median treatment duration was 13.8, 3.7, and 3.7 months for bevacizumab, carboplatin, and paclitaxel, respectively. Treatment-related Grade 3/4 adverse events occurred in 46 (94%) patients. Three (6%) patients died, all due to progressive disease.
Table 3. Summary of adverse events in the safety evaluable population*.
| Safety summary | Bev + CP (n=49) | Placebo + CP (n=50) | |
|---|---|---|---|
| Treatment duration (mo) | Bev: 13.8 (0–17) | Placebo: 9.0 (0–16) | |
| Carboplatin: 3.7 (2–6) | Carboplatin: 3.6 (1–5) | ||
| Paclitaxel: 3.7 (2–6) | Paclitaxel: 3.6 (0–5) | ||
| All-grade AEs, any cause | 49 (100.0) | 50 (100.0) | |
| Treatment-related AEs | 49 (100.0) | 50 (100.0) | |
| Grade 3/4 AEs* | 46 (93.9) | 37 (74.0) | |
| Treatment-related grade 3/4 AEs† | 46 (93.9) | 34 (68.0) | |
| Serious AEs | 17 (34.7) | 16 (32.0) | |
| Treatment-related serious AEs | 15 (30.6) | 15 (30.0) | |
| Grade 5 AEs | 0 (0.0) | 0 (0.0) | |
| Treatment-related grade 5 AEs | 0 (0.0) | 0 (0.0) | |
| AE leading to any study drug discontinuation | 3 (6.1) | 3 (6.0) | |
| AE leading to Bev/placebo discontinuation | 3 (6.1) | 1 (2.0) | |
| AE leading to carboplatin discontinuation | 0 (0.0) | 2 (4.0) | |
| AE leading to paclitaxel discontinuation | 0 (0.0) | 3 (6.0) | |
| AE leading to any dose reduction or interruption | 30 (61.2) | 30 (60.0) | |
| AE leading to dose interruption of Bev/placebo | 25 (51.0) | 20 (40.0) | |
| AE leading to dose reduction or interruption of carboplatin | 26 (53.1) | 26 (52.0) | |
| AE leading to dose reduction or interruption of paclitaxel | 24 (49.0) | 25 (50.0) | |
Values are presented as median (range) or number (%).
AE, adverse event; Bev, bevacizumab; CP, carboplatin plus paclitaxel.
*The safety evaluable population comprised patients who received any amount of any component of the study treatments (Bev, placebo, carboplatin, or paclitaxel). For the safety analysis, patients were allocated to treatment arms according to the treatment they actually received (i.e., patients randomized to the placebo + CP arm who received at least one full or partial dose of Bev were included in the Bev + CP arm).
†Refers to the highest grade experienced.
In the placebo + CP arm, median treatment duration was 9.0, 3.6, and 3.6 months for placebo, carboplatin, and paclitaxel, respectively. Treatment-related grade 3/4 adverse events occurred in 34 (68%) patients. Six (12%) patients died; 5 were due to progressive disease, and 1 due to advanced organ failure after disease progression.
Adverse events with the highest incidence in both the bevacizumab + CP and placebo + CP arms were alopecia (43 [88%] vs 41 [82%] patients), decrease in neutrophil count (39 [80%] vs 34 [68%]), anemia (38 [78%] vs 35 [70%]), and decrease in white blood cell count (38 [78%] vs 29 [58%]); Table S2).
Adverse events of special interest (AESIs) for bevacizumab occurred in 37 patients (76%; grade 3/4: 8 [16%] patients) in the bevacizumab + CP arm, and in 20 patients (40%; grade 3/4: 4 [8%]) in the placebo + CP arm (Table S3). Most AESIs were of grade 1/2 severity, and generally manageable by withholding bevacizumab and/or appropriate treatment. These AESIs were considered by the investigator to be treatment-related in 71% (n=35) of patients in the bevacizumab + CP arm, compared with 38% (n=19) in the placebo + CP arm.
5. Impact of coronavirus disease 2019 (COVID-19)
This study was under way when the World Health Organization declared the COVID-19 pandemic on March 11, 2020. No patients discontinued study treatment due to the pandemic, and no adverse events were reported that were consistent with a confirmed or suspected COVID-19 infection. However, 3 (6%) patients in the bevacizumab + CP arm and 2 (4%) in the placebo + CP arm had ≥1 major protocol deviation related to COVID-19 (including missed tumor assessments and missed lab test assessments). Overall, the impact of COVID-19 on study conduct and data collection was determined to be minor by the investigators, and there was no impact on the study results and conclusions.
DISCUSSION
The results of this study were consistent with those observed in the global GOG-0218 study, demonstrating a clinically meaningful improvement (10-month increase) in PFS, and a 70% reduction in the risk of disease progression or death in Chinese patients who received bevacizumab + CP vs placebo + CP. Subgroup analyses showed a similar trend of PFS improvement across disease characteristics in patients receiving bevacizumab + CP.
Although a numerically lower number of deaths occurred in the bevacizumab + CP arm, OS data were immature at clinical cutoff, and no conclusions on OS can be drawn. The difference in objective response rate (unconfirmed) was 3% between patients treated with bevacizumab + CP and placebo + CP. Notably, there was an imbalance between arms, with 28 (57%) patients in the placebo + CP arm having measurable disease at baseline, compared with 24 (47%) patients in the bevacizumab + CP arm. Although the presence of measurable disease was not a stratification factor in this study, this imbalance is unlikely to have impacted results, given that the results were stratified by FIGO stage and debulking status, a well-acknowledged prognostic factor in ovarian cancer. A prolonged duration of response was observed in the bevacizumab + CP vs placebo + CP arm (median duration of response: 16.1 vs 8.9 months). Generally, across the treatment period, similar percentages of patients in each arm reported a clinically meaningful improvement in patient-reported function, health-related QoL, abdominal pain, and bloating.
Exploratory subgroup analyses showed that the PFS benefit of bevacizumab + CP versus placebo was maintained in prespecified subgroups, including in patients with FIGO stage III optimally debulked disease and FIGO stage III sub-optimally debulked disease. In the FIGO stage IV subgroup, HR for PFS appeared to favor placebo + CP. However, these results are limited by the small sample size (8 patients in each arm) of the FIGO stage IV subgroup and the wide 95% CI range for HR.
Bevacizumab was well tolerated and safety data were consistent with its well-established safety profile, with no new safety signals identified in this study.
This study represents, to our knowledge, the first efficacy data from a randomized, placebo-controlled, phase III trial of first-line bevacizumab + CP vs placebo + CP in Chinese patients with ovarian cancer. The aim of this bridging study was to investigate whether the treatment effect of bevacizumab + CP was consistent with that observed in the global GOG-0218 study. These data support the conclusion that bevacizumab is efficacious as an ovarian cancer therapy across different races, and are consistent with results from the global GOG-0218 and ICON7 studies [8,10,11,12], as well as previous real-world evidence studies and clinical trials in predominantly Caucasian populations [14,15,16,17].
Bevacizumab + CP remains the standard of care as primary therapy following initial surgical resection in stage III–IV ovarian cancer [26], despite the emergence of PARP inhibitors. PARP inhibitors recently proved efficacious in a wide range of settings, including first-line maintenance in advanced ovarian cancer, recurrent ovarian cancer, and BRCA-mutated or homologous recombination deficiency-positive ovarian cancer after 3 or more prior lines of chemotherapy. Olaparib, niraparib and rucaparib are approved by the FDA for first-line maintenance treatment of advanced ovarian cancer, as well as for maintenance treatment of recurrent ovarian cancer [19,20,21]. In addition to these advances with PARP inhibitors alone, the combination of olaparib and bevacizumab is also approved by the FDA for first-line maintenance treatment in patients with a homologous recombination deficiency (either a BRCA mutation or genomic instability) [19].
Results from this study add to existing evidence that demonstrate the efficacy of bevacizumab + CP for first-line ovarian cancer treatment. Other recent studies, including the phase III MITO16b/MANGO–OV2/ENGOT–ov17 trial, demonstrate the efficacy of bevacizumab plus chemotherapy in the second-line ovarian cancer setting [18]. Second-line bevacizumab plus chemotherapy has been further evaluated in the phase III GOG0213 and OCEANS trials [28,29].
Since YO40268 used a bridging study design, it was not sufficiently powered for the primary endpoint (PFS); thus, data on subgroup analyses of PFS should be interpreted with caution due to the small sample size and wide 95% CIs. However, the study achieved its purpose of confirming the efficacy and safety of bevacizumab + CP in Chinese patients and showing the consistency in treatment effect of the bevacizumab + CP combination in Chinese patients compared with a global study population.
Owing to the short follow-up period at clinical cutoff, median OS was not reached, and the OS data were too limited to be included in this analysis. Nonetheless, this study sheds light on the efficacy and safety of bevacizumab + CP in Chinese patients with advanced ovarian cancer, showing the promise of this combination as first-line treatment for this population.
YO40268 is the first randomized study to investigate bevacizumab in Chinese patients with ovarian cancer and showed clinically meaningful improvement in PFS, corresponding to a 70% reduction in the risk of disease progression or death, with first-line bevacizumab + CP treatment. Safety data in this study were aligned with the known bevacizumab safety profile. These results support the use of bevacizumab + CP for the treatment of Chinese patients with previously untreated ovarian cancer and are pivotal for the full approval of bevacizumab for the treatment of ovarian cancer in China.
ACKNOWLEDGEMENTS
The authors would like to thank the patients who participated in the trial, the patients’ families, and the investigators and staff at all clinical study sites. Third-party medical writing assistance for this manuscript was provided by Akshaya Srinivasan, PhD, CMPP, of Nucleus Global, an Inizio Company.
Footnotes
Funding: This study and third-party medical writing assistance was sponsored by F. Hoffmann-La Roche Ltd.
Conflict of Interest: W.X., L.J., A.R., Y.R., Z.Y., Z.H., H.A., W.L., Z.J., L.Z., D.W., Z.J., L.G., C.G., C.Y., and X.F. received institute research funding from F. Hoffmann-La Roche Ltd. N.S., W.H., and L.D. are employees of F. Hoffmann-La Roche Ltd. N.S. and W.H. own shares in F. Hoffmann-La Roche Ltd.
Data Availability: Qualified researchers may request access to individual patient level data through the clinical study data request platform (https://vivli.org). Further details on Roche’s criteria for eligible studies are available at https://vivli.org/members/ourmembers. For further details on Roche’s Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, visit https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm.
- Conceptualization: W.H.
- Data curation: N.S., W.H., L.D.
- Formal analysis: L.D.
- Investigation: W.X., L.J., A.R., Y.R., Z.Y., Z.H., H.A., W.L., Z.J., L.Z., D.W., Z.J., L.G., C.G., C.Y., X.F.
- Methodology: L.D.
- Supervision: W.H.
- Validation: W.H., L.D.
- Writing - original draft: N.S., W.H., L.D.
- Writing - review and editing: W.X., L.J., A.R., Y.R., Z.Y., Z.H., H.A., W.L., Z.J., L.Z., D.W., Z.J., L.G., C.G., C.Y., X.F., N.S., W.H., L.D.
SUPPLEMENTARY MATERIALS
Supplementary methods
Patients with a clinically meaningful improvement (≥10 points difference) in patient-reported outcomes from baseline to end of treatment
Adverse events with an incidence rate of at least 10% in any treatment arm in the safety evaluable population*
AESIs* with Bev in the safety evaluable population†
Study design.
Screening, randomization and follow-up of study patients (intention-to-treat population).
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Cao W, Chen HD, Yu YW, Li N, Chen WQ. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J (Engl) 2021;134:783–791. doi: 10.1097/CM9.0000000000001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ebell MH, Culp MB, Radke TJ. A systematic review of symptoms for the diagnosis of ovarian cancer. Am J Prev Med. 2016;50:384–394. doi: 10.1016/j.amepre.2015.09.023. [DOI] [PubMed] [Google Scholar]
- 4.Torre LA, Trabert B, DeSantis CE, Miller KD, Samimi G, Runowicz CD, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68:284–296. doi: 10.3322/caac.21456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karam A, Ledermann JA, Kim JW, Sehouli J, Lu K, Gourley C, et al. Fifth Ovarian Cancer Consensus Conference of the Gynecologic Cancer InterGroup: first-line interventions. Ann Oncol. 2017;28:711–717. doi: 10.1093/annonc/mdx011. [DOI] [PubMed] [Google Scholar]
- 6.du Bois A, Reuss A, Pujade-Lauraine E, Harter P, Ray-Coquard I, Pfisterer J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire (GINECO) Cancer. 2009;115:1234–1244. doi: 10.1002/cncr.24149. [DOI] [PubMed] [Google Scholar]
- 7.Herzog TJ. Recurrent ovarian cancer: how important is it to treat to disease progression? Clin Cancer Res. 2004;10:7439–7449. doi: 10.1158/1078-0432.CCR-04-0683. [DOI] [PubMed] [Google Scholar]
- 8.Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365:2473–2483. doi: 10.1056/NEJMoa1104390. [DOI] [PubMed] [Google Scholar]
- 9.Presta LG, Chen H, O’Connor SJ, Chisholm V, Meng YG, Krummen L, et al. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 1997;57:4593–4599. [PubMed] [Google Scholar]
- 10.Perren TJ, Swart AM, Pfisterer J, Ledermann JA, Pujade-Lauraine E, Kristensen G, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365:2484–2496. doi: 10.1056/NEJMoa1103799. [DOI] [PubMed] [Google Scholar]
- 11.Tewari KS, Burger RA, Enserro D, Norquist BM, Swisher EM, Brady MF, et al. Final overall survival of a randomized trial of bevacizumab for primary treatment of ovarian cancer. J Clin Oncol. 2019;37:2317–2328. doi: 10.1200/JCO.19.01009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oza AM, Cook AD, Pfisterer J, Embleton A, Ledermann JA, Pujade-Lauraine E, et al. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): overall survival results of a phase 3 randomised trial. Lancet Oncol. 2015;16:928–936. doi: 10.1016/S1470-2045(15)00086-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Avastin prescribing information. South San Francisco, CA: Genentech, Inc.; 2020. [Google Scholar]
- 14.Mustea A, Wimberger P, Oskay-Oezcelik G, Jungberg P, Meinerz W, Reichert D, et al. Impact of age on the safety and efficacy of bevacizumab (BEV)-containing therapy in patients (pts) with primary ovarian cancer (OC): analyses of the OTILIA German non-interventional study on behalf of the North-Eastern German Society of Gynaecological Oncology Ovarian Cancer Working Group. Ann Oncol. 2016;27:vi299. [Google Scholar]
- 15.Nikolaou M, Ziras N, Athanasiadis I, Ardavanis A, Vaslamatzis M, Kentepozidis NK, et al. Real life efficacy and safety data of bevacizumab-based front line treatment in advance or metastatic ovarian cancer patients: focus on patients with malignant ascites – a phase IV study. J Clin Oncol. 2019;37(15_suppl):5562. [Google Scholar]
- 16.Hall M, Bertelli G, Li L, Green C, Chan S, Yeoh CC, et al. Role of front-line bevacizumab in advanced ovarian cancer: the OSCAR study. Int J Gynecol Cancer. 2020;30:213–220. doi: 10.1136/ijgc-2019-000512. [DOI] [PubMed] [Google Scholar]
- 17.Sehouli J, Mustea A, Oskay-Özcelik G, Keller M, Richter R, Tomé O, et al. Bevacizumab combined with platinum-taxane chemotherapy as first-line treatment for advanced ovarian cancer: results of the NOGGO Non-Interventional Study (OTILIA) in 824 patients. Cancers (Basel) 2021;13:4739. doi: 10.3390/cancers13194739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pignata S, Lorusso D, Joly F, Gallo C, Colombo N, Sessa C, et al. Carboplatin-based doublet plus bevacizumab beyond progression versus carboplatin-based doublet alone in patients with platinum-sensitive ovarian cancer: a randomised, phase 3 trial. Lancet Oncol. 2021;22:267–276. doi: 10.1016/S1470-2045(20)30637-9. [DOI] [PubMed] [Google Scholar]
- 19.Lynparza prescribing information. Wilmington, DE: AstraZeneca Pharmaceuticals; 2020. [Google Scholar]
- 20.Zejula prescribing information. Brentford: GlaxoSmithKline; 2020. [Google Scholar]
- 21.Rubraca prescribing information. Boulder, CO: Clovis Oncology Inc.; 2020. [Google Scholar]
- 22.Kurnit KC, Avila M, Hinchcliff EM, Coleman RL, Westin SN. PARP inhibition in the ovarian cancer patient: current approvals and future directions. Pharmacol Ther. 2020;213:107588. doi: 10.1016/j.pharmthera.2020.107588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chinese Society of Gynecological Oncology. Guidelines for clinical application of PARP inhibitors in ovarian cancer. Chinese J Front Med Sci. 2020;12:29–37. [Google Scholar]
- 24.Wang J, Zhu J. Real-world hematological adverse events in Chinese patients with advanced ovarian cancer treated with an individualized starting dose of niraparib. Ann Transl Med. 2021;9:869. doi: 10.21037/atm-21-2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fluzoparib China prescribing information. Lianyungang: Jiangsu Hengrui Pharmaceuticals Co.; 2021. [Google Scholar]
- 26.Colombo N, Sessa C, du Bois A, Ledermann J, McCluggage WG, McNeish I, et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Ann Oncol. 2019;30:672–705. doi: 10.1093/annonc/mdz062. [DOI] [PubMed] [Google Scholar]
- 27.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 28.Coleman RL, Brady MF, Herzog TJ, Sabbatini P, Armstrong DK, Walker JL, et al. Bevacizumab and paclitaxel-carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/Gynecologic Oncology Group study GOG-0213): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18:779–791. doi: 10.1016/S1470-2045(17)30279-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aghajanian C, Blank SV, Goff BA, Judson PL, Teneriello MG, Husain A, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2012;30:2039–2045. doi: 10.1200/JCO.2012.42.0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary methods
Patients with a clinically meaningful improvement (≥10 points difference) in patient-reported outcomes from baseline to end of treatment
Adverse events with an incidence rate of at least 10% in any treatment arm in the safety evaluable population*
AESIs* with Bev in the safety evaluable population†
Study design.
Screening, randomization and follow-up of study patients (intention-to-treat population).