ABSTRACT
Middle East respiratory syndrome coronavirus (MERS-CoV) is a lethal beta-coronavirus that emerged in 2012. The virus is part of the WHO blueprint priority list with a concerning fatality rate of 35%. Scientific efforts are ongoing for the development of vaccines, anti-viral and biotherapeutics, which are majorly directed toward the structural spike protein. However, the ongoing effort is challenging due to conformational instability of the spike protein and the evasion strategy posed by the MERS-CoV. In this study, we have expressed and purified the MERS-CoV pre-fusion spike protein in the Expi293F mammalian expression system. The purified protein was extensively characterized for its biochemical and biophysical properties. Thermal stability analysis showed a melting temperature of 58°C and the protein resisted major structural changes at elevated temperature as revealed by fluorescence spectroscopy and circular dichroism. Immunological assessment of the MERS-CoV spike immunogen in BALB/c mice with AddaVaxTM and Imject alum adjuvants showed elicitation of high titer antibody responses but a more balanced Th1/Th2 response with AddaVaxTM squalene like adjuvant. Together, our results suggest the formation of higher-order trimeric pre-fusion MERS-CoV spike proteins, which were able to induce robust immune responses. The comprehensive characterization of MERS-CoV spike protein warrants a better understanding of MERS spike protein and future vaccine development efforts.
KEYWORDS: MERS-CoV vaccine; prefusion spike; immunogenicity; thermostability, antibody response
Introduction
The current pandemic of SARS-CoV-2 has reminded the world of the potential threats of respiratory diseases and the need for pre-pandemic preparation.1 One of the epidemics caused by MERS-CoV was originated in Saudi Arabia in the year 2012 due to the spill over from dromedary camels to humans.2 The causative agent was identified as the Middle East Respiratory Syndrome Coronavirus (MERS-CoV), a lineage C betacoronavirus, which soon spread across 27 countries.3 It had an exceptionally high fatality rate of 35% and continues to cause sporadic outbreaks in Saudi Arabia as well as other countries largely due to international travel.3 In the Korean outbreak, the largest outside the Middle East, a single affected patient led to over 180 infections along with 36 deaths.4 As of May 2023, 2604 cases with 936 deaths have been reported worldwide.5 The clinical features vary from asymptomatic or flu-like to severe pneumonia, and acute respiratory distress leading to multi-organ failure and death. Currently, there are no approved anti-viral therapeutics available to treat MERS-CoV, although many vaccine candidates are either in the pre-clinical or in advanced clinical study.6
MERS-CoV is an enveloped virus, consisting of a single, positive-stranded RNA, and similar to other corona viruses the structural envelope spike (S) glycoprotein is the key structural protein which is engaged in the virus entry. The spike glycoprotein binds to the host receptor dipeptidyl peptidase-4 (DPP4), thus facilitating the fusion and virus entry.7 The spike glycoprotein also induces neutralizing antibodies in naturally infected individuals. Thus, the spike glycoprotein is the major target for vaccine development.8 MERS-CoV S protein is 1353 amino acids long and consists of two important subunits, the N-terminal S1 domain, responsible for binding to the receptor through the receptor binding domain (RBD), and the C – terminal S2 domain which induces the fusion with the host cell membrane.8 The S protein is a class I protein that is present on the viral surface as a homo-trimer, which undergoes an extensive conformational change from pre-fusion to post-fusion state upon binding to the host cell receptor,9–11 and key determinant of virus-induced infection and subsequent progression of pathogenesis. However, unlike the rapid development of the SARS-CoV-2 vaccine, the MERS-CoV vaccine development is still under progress, and a few products have either entered or completed the Phase-I clinical trial.12–14 The spike or RBD domain has been the focus of research efforts to create a vaccine against MERS-CoV; however, a number of obstacles have made progress in this area slow and inconsistent. The unavailability of proper animal models, sequence variability, the threat of disease enhancement as shown in SARS-CoV,15 and limited understanding of vaccine-induced protection or long lasting immune responses are some of the challenges that need to be considered for an ongoing process of MERS-CoV vaccine development.16,17
In this study, we have designed a pre-fusion MERS-CoV spike trimer protein (M-CoV-S), expressed in mammalian suspension culture using Expi293F cells, and extensively characterized its physiochemical properties. The immunogenicity assessment in the mice showed that the spike immunogen M-CoV-S in the presence of either AddaVaxTM or Imject alum adjuvant was able to elicit high titer antibody responses. Additionally, the structural binding studies of the MERS-CoV-S protein docked with the MCA-1 neutralizing antibody showed similar binding area as it has been reported previously 6GMQ (interacting with RBD).18 Taken together, data from the current study enhances the understanding of MERS-CoV synthetic trimeric spike protein oligomers as a promising immunogen, and its conformational plasticity which might be the major dominant factor in eliciting immune responses.
Materials and methods
Cloning, expression, and purification of MERS-CoV prefusion spike ectodomain from Expi293F cells
A human-codon-optimized gene encoding MERS-CoV spike (England 1 strain, YP_009047204.1) from residues 16–1291, flanked by a signal peptide at the N-terminal and with a T4 fibritin trimerization domain and 8×His-tag at C-terminal, was synthesized and subcloned into the eukaryotic expression vector pDNA 3.1 with restriction sites for BamHI and XbaI.19 The S1/S2 furin-recognition site 748-RSVR-751 was mutated to ASVG to produce a single-chain S0 protein resembling the prefusion molecule. Along with this, two proline mutations at residues V1060 and L1061 were introduced for stabilization in the recombinant construct. Plasmids encoding human codon-optimized MERS-CoV spike were synthesized by Thermo Fisher Scientific, USA (Life Technologies Corporation, USA).
For suspension cell culture, Expi293F cells were maintained in suspension cultures in Expi Expression Medium ((#A1435101, Thermo Fisher Scientific, USA) and transfection was conducted using the ExpiFectamine 293 Transfection Kit (#A14524, Thermo Fisher Scientific) according to the manufacturer’s instructions. The supernatant was harvested after 5–7 days of post-transfection or when the viability reached < 60%. Cultures were centrifuged and supernatant filtered to remove cellular debris before IMAC protein purification using Ni-NTA resin (#30210, QIAGEN, India) and an automated AKTA purification system (Akta Pure 25, Cytiva lifesciences). The eluted protein was then dialyzed against PBS at 4°C and thereafter concentrated with a 50 ml amicon filter with a 30K cutoff to get the final protein. The purity of purified M-CoV-S protein was analyzed by 12% resolving gel on SDS-PAGE and further staining by Coomassie blue stain.
Western blotting and blue native gel analysis
The purified M-CoV-S protein was resolved on a 12% SDS-PAGE and transferred to a Millipore polyvinylidene fluoride (PVDF) membrane. The membrane was blocked in blocking buffer for 2 hours at 37°C followed by incubation with anti-MERS-CoV-S mouse polyclonal sera (1:5000) and commercial MERS-RBD (#10621-CV, R&D Systems) (1:1000) at 4°C for overnight. The membrane was washed with washing buffer (0.1% Tween 20 in PBS) and further developed with anti-mouse/anti-human HRP conjugated secondary antibody (#115-035-003, Jackson Immuno Research, USA) at a dilution of 1:5000 was added and then the mixture was incubated for 1 hour at 37°C. Later, the membrane was washed twice with wash solution and lastly washed with PBS. The blot was then developed using an ECL reagent (#786–003, G-Biosciences) and imaged using Bio rad Chemidoc.
For Native-PAGE About 60 µg of protein was diluted in commercial 4× Native dye (no heating) and loaded onto a precast native gel (4–15%) (Invitrogen™ and BioRad pre cast gel). 0.05% Coomassie G250 in Native PAGE running buffer was used as cathode buffer. The protein was resolved at 120 V for 3 hours. The gel was stained in Coomassie stain for 1 hour followed by de-staining.
Size exclusion chromatography
The purified M-CoV-S protein was run on the Superdex 200 Increase column for size exclusion chromatography. The column was equilibrated with 3 CV (column volume) of equilibration buffer (10 mM PBS). The M-CoV-S protein (3 mg/ml) was injected manually using a capillary loop. 1.5 CV of 10 mM PBS (flow rate of 0.750 ml/min) was used to elute the protein. UNICORN 7.1 software was used to analyze the chromatogram.
Differential scanning colorimetry
The melting temperature of M-CoV-S protein was estimated using Nano-DSC (TA instruments). M-CoV-S protein in 10 mM PBS at a concentration of 0.5 mg/ml was loaded onto the instrument against 10 mM PBS as a reference. A scanning rate of 1 °C/min under 3.0 atmospheres of pressure was used to perform thermal melting. Data collected was then analyzed using NanoAnalyze software, 3.6.0 (TA instruments), and the graph plotted using Graph Pad Prism.
Fluorescence spectroscopy
A Varian Fluorescence spectrophotometer was used to measure the tryptophan fluorescence of the M-CoV-S. M-CoV-S was excited at 280 nm wavelength and emission spectra were recorded from 290 nm to 500 nm with a slit width of 5 nm. Three independent spectra were recorded and temperature was maintained by the attached Peltier temperature controller. Further, for thermal analysis the temperature was increased,20,21 and emission spectra were recorded for each temperature.
FAR UV circular dichroism
Circular dichroism (CD) was used to monitor the secondary structure. All the major secondary structures of protein have a characteristic CD spectrum. M-CoV-S prefusion spike at a concentration of 200 µg/ml was used for CD spectroscopy using JASCO 815 spectrometer, Japan. The instrument was flushed with nitrogen for 2 minutes and the lamp was allowed to warm for 10 minutes. The temperature controller was set to 4°C. Spectrum was recorded at a bandwidth of 1 nm with a step size of 1 nm. Three measurements were made and then Yang’s reference spectra were used to estimate the relative proportions of different secondary structures using Spectra Manager software from JASCO. Further, for thermal analysis, the temperature was increased to respective temperature,20 ,21 and spectra were recorded.
Size and zeta potential
M-CoV-S spike was characterized for size, polydispersity index, and zeta potential using Zetasizer Nano ZS (Malvern Instruments Ltd., U.K.). 1 mg/ml of protein in disposable sizing plastic cuvette was used for measurement and disposable folded capillary cell for zeta potential. Reading was represented as the average of three independent measurements.
Ethics statement and mice immunization
All animal experimentation was conducted in compliance with the ethical considerations and guidelines issued by the Committee for Control and Supervision of Experiments on Animals (CPSEA) of the Government of India and with the approval of the Institutional Animal Ethics Committee (IAEC, THSTI) regarding laboratory animals (IAEC Approval number: IAEC/THSTI/256). For the immunization study, 6–8 weeks old female BALB/c mice weighing 18–25 g and inbred in the institute’s (THSTI) experimental animal facility (EAF) were used. 29 mice were randomly divided into five groups having six mice in each group. All the immunizations administered were investigator blindfolded. One group was immunized with M-CoV-S + AddaVaxTM (#vac-adx-10, Invivogen) as adjuvant mixed in a 1:1 ratio containing 25 μg of protein. The second group (Addavax control group) was treated with PBS + AddaVaxTM mixed in a 1:1 ratio. The third group was immunized with M-CoV-S + Imject adjuvant (#77161, Thermo Fisher Scientific) mixed in a 1:1 ratio containing 25 μg of protein. The fourth group (Imject control group) was treated with PBS + Imject mixed in a 1:1 ratio. Fifth group was treated with PBS only. Immunization was performed via the intramuscular route (cranial thigh muscles) twice at an interval of 4 weeks in a prime-boost regimen. The mice were observed daily and weighed twice a week over the course of the immunization period. Blood sample from each mouse was collected at day 0 (pre-immune sera), day 14 (sera after priming), day 14 and day 21 (sera after the boost). The serum was separated from the blood, heat inactivated at 56°C for 30 min and stored at − 20 ◦C for future use.
Enzyme Linked immunosorbent assay (ELISA)
The ELISA was performed to characterize the binding of the IgG and its subtypes from immunized sera collected from MERS-CoV prefusion spike protein groups. The M-CoV-S protein was coated on Maxisorp plates (#M9410, Nunc, Sigma Aldrich, India) with 100 μl of protein at 2 μg/ml concentration in 1×carbonate/bicarbonate buffer, pH 9.6 for overnight at 4°C. Next day, the plate was blocked by adding 200 μl/well of PBS containing 5% skimmed milk. Immunized mice sera collected from all six mice from each group were added separately in three-fold dilutions to the wells and incubated for 1 hr at RT. The wells were washed and HRP-conjugated anti-mouse secondary antibody (#115-035-003, Jackson Immuno Research, USA) in 1:2000 dilutions were used for developing ELISA. Similarly, for IgG subtyping, in other round of experiments secondary antibodies against IgG1(#115-035-205), IgG2a (#115-035-206), IgG2b (#115-035-207), and IgG3 (#115-035-209) were used as secondary antibodies, the plates were developed with tetramethylbenzidine (TMB) (#002023, Sigma-Aldrich, India) substrate, and the reaction was allowed to develop for 5-min, and then stopped with 1N sulfuric acid. The absorbance of plates was recorded at 450 nm on an ELISA reader (Bio-Rad). The endpoint titer was determined for IgG and each subtype which is the reciprocal of the highest test serum dilution giving a reading above the cutoff. In each assay, the sera from PBS treated mice was considered as negative control to calculate the cutoff value. The cutoff value was calculated by multiplying the average OD value of negative control samples + 3 times SD of OD value of negative control samples. The test result was considered positive when signal to cutoff ratio >/ = 1.
Immune profiling of the serum cytokines
Th1/Th2 cytokine levels were measured in immunized (M-CoV-S with AddaVaxTM or Imject) and non-immunized control mouse serum collected after the booster dose (day 42) using a Mouse Th1/Th2 CBA kit (#560485, BD Biosciences, USA). The standard dilutions of cytokines and test samples were incubated with cytokine capture beads and PE-detection reagent at room temperature for 3 hours in the dark conditions. After the incubation, beads were washed twice with the wash buffer and resuspended in the wash buffer for data acquisition using BD FACS Canto II Flow Cytometer (BD Biosciences, CA, USA). Instrument setting for sample acquisition was done prior to the experiment as per the manufacturer’s instructions. The data analysis was performed using the software FCAP Array and graphs were plotted on GraphPad Prism 9.0 software.
Protein’s de-glycosylation with PNGase F
De-glycosylation of M-CoV-S protein by PNGase F (#A39245, Gibco) (non-denaturing reaction conditions) was used following the manufacturer’s protocol. Briefly, Master Mix was prepared by combining 4 µL of PNGase F Buffer 10X and 0.5 µL of PNGase F enzyme. The Master Mix was added to 25 µg of purified dialyzed prefusion M-CoV-S protein and reaction mixture was incubated at 50°C for 1 h. Along with PNGase F treated protein untreated protein was also run as control to determine the extent of de-glycosylation. PNGase F treated and untreated samples were run on 12% SDS-PAGE and the extent of de-glycosylation was estimated by the shift in mobility of protein bands.
Immuno-fluorescence analysis
BHK21 cells (40,000/well) (ATCC) were seeded in Minimum Essential medium Eagle (MEM from Himedia) containing 10% FBS and 100 U/ml Penicillium-Streptomycin (P/S from Gibco). Next day, 0.5 μg each of CoV-1 spike full-length plasmid and ACE2 expressing plasmid were co-transfected using FuGENE HD transfecting reagent (#E2311, Promega). After 24 hr of transfection, cells were fixed in 4% paraformaldehyde (PFA) for 15 mins and permeabilized with 0.1% Triton-X100. Nonspecific binding was blocked using 4% Fetal Bovine Serum in PBS for 1 hr at RT. Cells were then incubated at RT for 1 h with heat inactivated pooled serum samples from a terminal bleed of M-CoV-S immunized mice (day 49 after prime immunization) or the control group in 1:200 dilutions. After incubation, cells were washed and incubated for 1 hr at RT with the Alexa Fluor 488 (#115-545-062, Jackson Immuno Research, USA) labeled rabbit anti-mouse IgG secondary antibody (1:1000 dilutions). Cells were washed three times with PBS and their nuclei were counterstained with Hoechst 33,342 (Thermo Scientific) and images were acquired on an Olympus IX-71 fluorescence microscope.
For the immunofluorescence assay with influenza virus, The MDCK-London cells were infected with 10 TCID50 of the A/Guangdong-Maonan/SWL1536/2019 of influenza virus. After 36 hr of post-infection, the cells were fixed in 4% paraformaldehyde for 15 minutes and processed similarly as described above.
Structural modelling of the pre fusion M-CoV-S immunogen
The secondary structure of the pre-fusion M-CoV-S immunogen was determined using the PSIPRED server and SWISS-MODEL-Expasy.22 The PSIPRED server helps in predicting the basic building blocks of protein structures such as beta-strands, alpha helices, and coils by employing a network called feed-forward neural networks.23 The SWISS-MODEL-Expasy web server aids in performing homology modeling of the query sequence to the target sequence. The same sequence is further analyzed using the MEMSTAT-SVM server to predict the various structural domains present in the sequence of the M-CoV-S protein.
Homology modelling
The homology modeling of the MERS spike protein was carried out using the Prime module within the Schrödinger Release 2023–3 software. The protein sequence of MERS was subjected to NCBI BLASTp against the PDB database, and PDB ID: 6NB324 was identified as the most suitable template due to its high sequence identity. The three-dimensional crystal structure was retrieved from the RCSB PDB database and utilized as the template in the Schrödinger Prime modeling interface. Subsequent refinement involved loop refinement and energy minimization using the OPLS4 force field in Schrödinger Prime. The resulting minimized model underwent validation through Ramachandran plot and by energy minimization analysis.25
Protein preparation and docking
For protein preparation and docking, the structure with PDB ID 5GMQ representing MERS-CoV-RBD (resolution: 2.70 Å) was processed using the Protein Preparation Wizard of Schrödinger. The MERS-CoV-RBD region was extracted from the structure, and the MCA 1 antibody was pre-processed in the Protein Preparation Wizard. This involved the addition of hydrogens and bond orders, filling missing side-chains and loops using Prime, and capping at N- and C-terminals. Hydrogen bond optimization and restrained minimizations were performed for the systems using the OPLS4 force field,25–28 The prepared antibody structure was then docked to the modeled spike protein using PIPER module of Schrodinger, with non-CDR regions of the antibody being masked during the process. The CDR region was defined according to the Chothia definition.
Statistical analysis
All immunization experiments were carried out with (n = 6) BALB/c mice per group. ELISA was used for determining the antibody generated and was expressed as endpoint titer measured at a wavelength of 450 nm. Each mouse was treated as an individual sample and the endpoint titer was expressed as group mean. For ELISA, the statistical significance of group means and standard deviation (SD) was assessed by an unpaired students t-test where M-CoV-S + Addavax was compared with Addavax control and M-CoV-S + Imject was compared with Imject control groups. For analyzing serum cytokine levels, one-way ANOVA analysis was performed using the Tukey-Kramer test for multiple comparisons, followed by post hoc tests inbuilt in GraphPad Prism 9 (GraphPad Software Inc., San Diego, CA, USA) to test for normal distribution of the samples by the Shapiro-Wilk normality test. Statistical analysis was then performed using multiple comparison tests where necessary. Where, *p < .05, **p < .01, ***p < .001, and ****p < .0001 were considered significant.
Results
In vitro expression and purification of recombinant prefusion MERS-CoV spike protein
The MERS-CoV pre-fusion construct was designed by selecting the residues from 16–1291. At the C-terminus, the transmembrane region at residue 1292 was removed to avoid membrane anchoring and replaced by T4 fibritin trimerization domain for efficient trimer formation followed by 8× HisTag for IMAC based protein purification. The S1/S2 furin cleavage site 748-RSVR-751 was mutated to ASVG to produce a single-chain S0 protein and two proline mutations (V1060P and L1061P) in the HR2 domain were introduced to stabilize the pre-fusion conformation. The human codon optimized for mammalian expression of the full-length construct was commercially synthesized and cloned in eukaryotic expression vector pCDNA 3.1 (Supplementary Figure S1, Figure 1a). In the suspension cell culture, Expi293F cells were then transfected with the cloned plasmid, and cells were harvested 5–7 days of post-transfection. The protein was purified from the cell culture using Ni-NTA affinity chromatography. The purified MERS-CoV pre-fusion spike (M-CoV-S) protein was resolved in the 12% reducing SDS-PAGE as a single ~ 180 kDa band (Figure 1b). The presence of single band in the SDS-PAGE confirms un-cleaved precursor spike protein. Additionally, the expression of in-house produced M-CoV-S protein was also detected using the in-house M-CoV-S immunized polyclonal MERS sera by western blotting (Figure 1c, left lane). The M-CoV-S immunized mice serum was also used to detect commercial MERS-RBD (R&D systems (Figure 1c Lane 3). Next, the protein was assessed for native conformation by blue native PAGE. The protein resolved as a single prominent band at a higher molecular weight of ~ 1045 kDa (Figure 1d), thus indicating the formation of a homogeneous higher order oligomeric structure possibly a dimer-trimers. The protein was purified in three consistency batches and the yield was found to be ~10 mg per liter of expression media. We further assessed the presence of glycosylation of the M-CoV-S immunogen when expressed in mammalian suspension culture (Figure 1e). M-CoV-S protein contains ~ 25 N-glycosylation sites.9,29 N-glycosylation not only helps the viral proteins for the correct folding and transport, but also influences infectivity and immune responses.30 We found reduction on molecular weight or increase mobility of the M-CoV-S proteins in the SDS-PAGE after PNGase-F treatment, thus suggesting efficient removal of N-glycans from the M-CoV pre-fusion spike protein and indirectly confirming the presence of the N-glycosylation in the synthetic pre-fusion MERS-CoV spike proteins.
Figure 1.
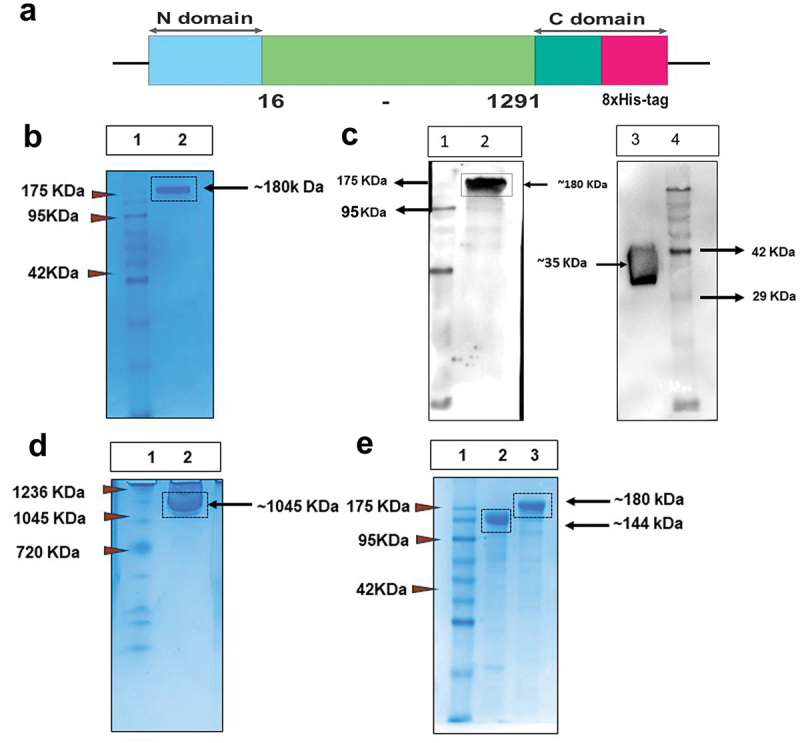
Expi293F based mammalian expression system produced soluble trimeric prefusion MERS-CoV Spike. (a) schematic representation of color-coded expression cassette used in mammalian (pcDNA3.1 (1)) expression vector; blue box represents signal peptide sequence at N-terminal domain for mammalian expression; light-green box represents human-codon-optimized gene encoding M-CoV-S sequences from 16–1291 amino acids taken from England 1 strain; and at C-terminal domain dark-green box represents T4 fibritin trimerization domain and pink box for 8×His-tag. (b)12% reducing SDS-PAGE of purified M-CoV-S (Lane1: Marker, Lane 2: M-CoV-S). (c) Western blot image of inhouse M-CoV-S and commercial MERS-RBD (#10621-CV, R&D systems) probed with anti-M-CoV-S mouse polyclonal serum (Lane 1, 4: Marker, Lane 2: In-house M-CoV-S, Lane 3: recombinant MERS-RBD). (d) Blue native-PAGE image of purified M-CoV-S protein. Lane 1: native Marker, Lane 2: native M-CoV-S. (e) Purified M-CoV-S protein was subjected to de-glycosylation by using PNGase F enzyme (Lane 1: Marker, Lane 2: PNGase treated M-CoV-S, Lane 3: untreated M-CoV-S).
Comprehensive biophysical characterization of M-CoV-S
The properties of transiently expressed soluble M-CoV-S proteins were further assessed by size exclusion chromatography (SEC) using the Superdex 200 column. Size exclusion chromatogram of the recombinant protein showed a single elution peak at 9 ml which indicates the formation of higher order oligomeric structure (Figure 2a). The combined data from the blue native-PAGE and size exclusion chromatography suggests the formation of a homogeneous dimer of trimers. Next, the size distribution profiling of a M-CoV-S protein was determined using dynamic light scattering (DLS) at room temperature with light scattering at 90° for individual measurement and observed to be 33.96 ± 1.20 nm with a PDI value of 0.265 (Figure 2b). The hydrodynamic volume measured using DLS for SARS-CoV-2 trimeric spike protein from different strains varies from 13–18 nm. The 34 nm size seen in case of M-CoV-S further suggests formation of dimer of trimers.31 Also, a PDI value of 0.25 indicates a uniform distribution of particle sizes of the synthesized M-CoV-S proteins in suspension. Further, the apparent zeta potential of a M-CoV-S protein was determined in the 10 mM PBS (pH 7.5). The magnitude of the zeta potential is significantly influenced by the ionic strength and pH of the dispersant. The purified M-CoV-S proteins were negatively charged and the apparent zeta potential was depicted to be ‒7.3 ± 2.20 mV and remained constant for repeated batches suggesting moderate to good colloidal stability of the synthesized protein.
Figure 2.
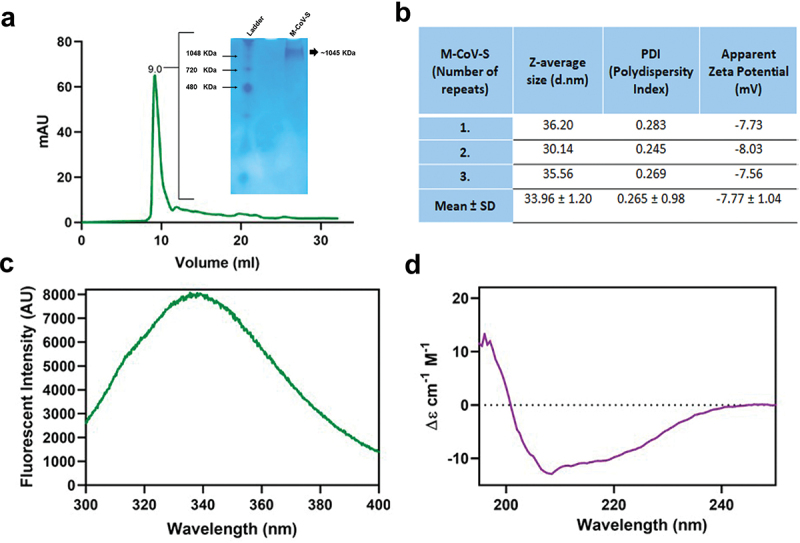
Biophysical characterization of purified M-CoV-S protein. (a) Size exclusion chromatography using superdex 200 increase. The purified M-CoV-S was injected manually using a 500 µl capillary loop. The column pre-equilibrated with 10 mM PBS was eluted with 1.5 CV of at flow rate of 0.750 ml/minute. The protein eluted at 9.0 ml. The inset graph shows the blue-native PAGE image of M-CoV-S protein after purification eluted at an size of ~ 1045 KDa. (b) Hydrodynamic size and apparent zeta potential measurement of M-CoV-S using Malvern Zetasizer. (c) Intrinsic tryptophan fluorescence of M-CoV-S. 1 mg/ml M-CoV-S was excited at wavelength of 280 nm and emission spectra acquired (300–400 nm). An emission maximum was recorded at 336 nm. (d) Far UV circular dichroism spectroscopy of M-CoV-S (250 µg/ml) in the range 195–250 nm range.
Next, the intrinsic fluorescence of M-CoV-S was measured using Varian Fluorescence spectrophotometer. The intrinsic fluorescence of a protein is a representative of the cumulative fluorescence due to all the fluorescence amino acids (mainly tryptophan and partially tyrosine residues). The Trp fluorescence maximum and intensity are highly influenced by the polarity of its micro-environment, hydrogen bonding and other non-covalent interactions that are present in a particular conformation. M-CoV-S pre-fusion spike contains 8 Trp and 75 Tyr residues. The emission maximum of fluorescence was recorded at a wavelength of 336 nm when taken at 4 degrees (Figure 2c). For the SARS-CoV-2 spike protein the reported emission maximum is at 325 nm. SARS-CoV-2 has 12 Trp and 55 Tyr residues while M-CoV-S has 8 Trp and 75 Tyr residues. The red shift in case of M-CoV-S may be accounted for the lower number of Trp and higher number of Tyr residues.31 Nevertheless, a good intensity of emission suggests that the overall M-CoV-S spikes’ structure is folded.32
M-CoV-S was further characterized for secondary structure by far UV circular dichroism (CD) spectroscopy. CD exploits the ability of optically active moieties present in proteins to absorb circularly polarized light differentially. The CD signature of M-CoV-S, which is very similar to that of SARS-CoV-2 spike protein,31 suggests equal percentage of both alpha-helix and beta-sheet structure, with slightly higher content of alpha-helix as modest negative peak can be seen at 222 nm and 208 nm. The similarity in CD signature of SARS-CoV-2 and MERS-spike protein may be attributed to similar geometry and content of the secondary structures in both the spike proteins. We used Spectra Manager Suite (Jasco) to deduce the secondary structure. It was observed that M-CoV-S showed a secondary structure consisting of 24% alpha-helical, 12% beta-sheet, and 35% random coil (Figure 2d).
M-CoV-S immunogen shows promising structural stability
The in-silico prediction of secondary structure derived from the M-CoV-S protein sequence using the PSIPRED server showed the presence of three building blocks of a protein structure such as helices, coils, and strands. In the M-CoV-S sequence of 1318 amino acids, in-silico prediction depicted a percentage of 14.32, 20.62, and 39.97 for alpha-helices, beta-strands, and coils, respectively (Supplementary Figure S2a). The sequence plot generated via MEMSTAT-SVM evaluated three different domains naming two extracellular domains (one from 1–260 amino acids and another from 950–1318 amino acids), one cytoplasmic domain (278–931 amino acids), and two short membrane-interacting domains (one from 261–277 amino acids and another from 932–949 amino acids) in the sequence of 1318 amino acids for the purified M-CoV-S proteins (Supplementary Figure S2b).
To experimentally assess the thermal stability and thermal unfolding of the spike protein, a differential scanning colorimetry (DSC) experiment was performed. DSC is a technique that measures the change in heat required to unfold a protein as a function of temperature. Thus, it is used to estimate the enthalpy for the process and thereby, the melting temperature (Tm) estimation of the protein. The M-CoV-S showed a single transition peak at 58°C (Figure 3a). Reports for stable forms of the SARS-CoV-2 spike proteins also recorded a single transition.21,33 We hypothesize that formation of dimer of trimers of M-CoV-S might have stabilize the structure and noncooperative unfolding among the different species (monomer, trimer, dimer-of-trimer) has obscured the transitional Tm of each subunit as a single transitional peak with Tm at 58°C. Additionally, the thermal stability of tertiary conformation was evaluated with SDS_PAGE and intrinsic tryptophan fluorescence as a function of temperature. The protein was stored at different temperatures (4°C, 25°C and 37°C) and assessed by SDS-PAGE. A single band was observed indicating that protein did not undergo any significant conformational change till one week at a temperature of 37°C (Figure 3b). Next, we observed, with increasing temperature, the fluorescence gradually decreased, however, the maxima of 336 nm were retained till a temperature of 37°C. At 55°C occurrence of a second maxima along with significant decrease in intensity was observed (Figure 3c). This indicates destabilization of protein structure was accompanied by tryptophan being exposed to a more hydrophilic environment, however, as prefusion spike contains 75 tyrosine along with 8 tryptophan, it may be due to self-quenching or tendency to retain the native structure. A gradual decrease in intensity but retention of maxima suggests that though temperature caused destabilization but protein had a tendency to retain its folded structure. We next used the far UV-CD spectra to study the temperature-dependent changes in the secondary structure of M-CoV-S protein. The CD spectrum remained intact till a temperature of 55°C. Analysis showed that M-CoV-S possessed 20–24% helix, 10–14% beta, and 30–35% random coil conformation up till a temperature of 37°C. Beyond 55°C there was a gain of 200 and 219 peaks indicating beta structure occurrence (Figure 3d).
Figure 3.
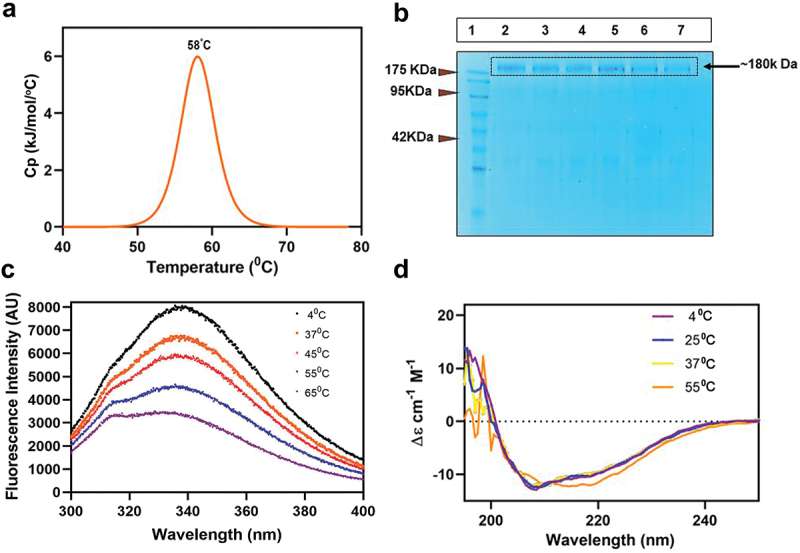
Thermal stability analysis of purified M-CoV-S. (a) differential scanning colorimetry for estimation Tm. M-CoV-S (0.5 mg/ml) was loaded onto nano-DSC with 10 mM PBS as a reference. Thermal melting at scanning rate of 1 °C/min and 3.0 atmospheres of pressure. NanoAnalyze software showed a Tm of 58°C. (b) SDS-PAGE analysis of M-CoV-S stored at different temperatures. Lane 1: Marker, lane 2: 4°C day 3, lane 3: 4°C day 7, lane 4: 25°C day 3, lane 5: 25°C day 7, lane 6: 37°C day 3, lane 7: 37°C day 7. (c) Intrinsic tryptophan fluorescence of M-CoV-S at different temperatures. (d) Far UV circular dichroism spectroscopy of M-CoV-S at different temperatures.
M-CoV-S in combination with either AddaVaxTM or imject adjuvants induces high titer humoral immune response
Adjuvants play an important role in stimulating and enhancing the immune responses mainly in protein-based vaccine formulation.34,35 Hence, to evaluate the effect of M-CoV-S antigen to elicit immune responses in the presence of adjuvants, we included two key adjuvants that are licensed for human use; a) AddaVaxTM, which is MF59 squalene-like adjuvant, known to induce a balanced Th1/Th2 responses, and b) Imject an Alum adjuvant showed to have enhanced immune responses and induces Th2 type responses.36 As described in materials and methods BALB/c mice were immunized intramuscularly in prime-boost regimen with 25 µg of M-CoV-S antigen either with AddaVaxTM or Imject adjuvant (Figure 4a). It was observed that after one immunization of prime dose, the immunized mice with M-CoV-S antigen + AddaVaxTM elicited moderately higher (206550) whole IgG titer than the M-CoV-S antigen + Imject immunized group (6750) (Figure 4c) p < .0048. However, after boosting, both the immunized groups elicited high antibody responses as measured at 14 days (mean of 984,150 for both the M-CoV-S adjuvanted groups) and 21 days post-boosting immunized sera (mean of 984,150 for M-CoV-S addavax and 874,800 for M-CoV-S Imject group, p < .3409) (Figure 4c-e) as compared to the control mice immunized with only adjuvants. The assessment of subtypes of IgG was estimated for the day 14 boost serum. It was observed that both the adjuvants generated similar IgG1 and IgG2b titers (Figure 4f). Although, the immunized AddaVaxTM group showed higher IgG2a responses whereas Imject immunized group showed higher IgG3 titer, suggesting that AddavaxTM adjuvant induced a more balanced Th1/Th2 response compared to the Imject adjuvant in combination with MERS pre-fusion spike protein.
Figure 4.
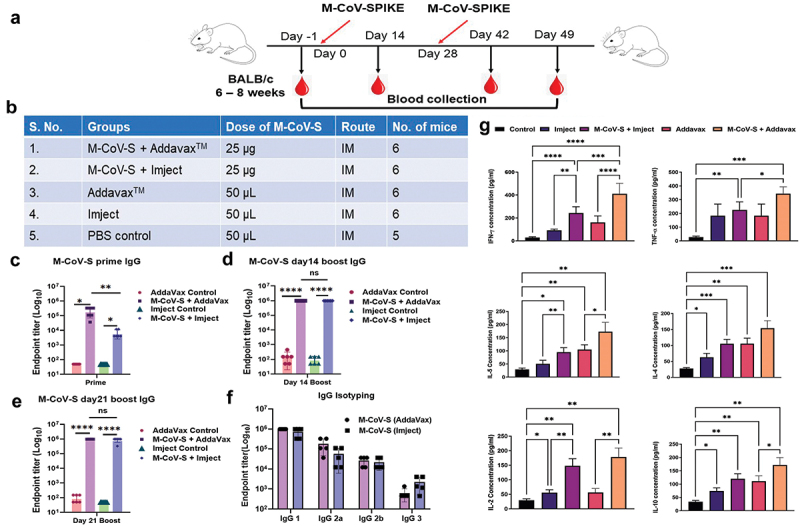
Evaluation of Immunogenic potential of MERS-CoV prefusion spike given with either AddaVaxTM or imject adjuvants. (a) 6–8 weeks old BALB/c mice were immunized with M-CoV-S prefusion protein either with addavax or imject in a prime-boost regimen at 28 days apart. Serum sample was harvested on 14 days after prime and day 14 and day 28 after boosting. Serum was separated, heat inactivated and stored at − 20°C and was used for IgG and its subtypes endpoint titer estimation. (b) Table describing different groups, dose of M-CoV-S immunogen and route of immunization. 29 female BALB/c mice were divided in to six groups having 6 mice each except PBS control group had 5 mice. (c) Total IgG end point titer estimation of prime (day 14) serum by homologous protein. (d) IgG estimation at day 14 post boost. (e) IgG estimation day 21 post boost. (f) IgG subtyping using anti-mouse HRP conjugated secondary antibodies (IgG1, IgG 2a, IgG 2b, and IgG3). (g) Th1/Th2 cytokines level was estimated in boost serum using the cytometric bead array. The values shown in the graphs are the geometric mean titers ± S.E. of triplicate wells. The statistical significance of the experiment was calculated between two groups by students t-test and between more than two groups with the one-way ANOVA test (Tukey-Kramer test). Where, *p < .05, **p < .01, ***p < .001, and ****p < .0001 were considered significant.
Next, we assessed the immune profiling of the serum collected from the MERS-CoV spike immunized mice after 14 days of booster dose using the Th1/Th2-Mouse CBA kit. We determined the serum concentration of seven different cytokines i.e., IFN-γ, TNF-α, IL-5, IL-4, IL-17a, IL-10, and IL-2 (Figure 4g). The serum concentration of IFN-γ, TNF-α, and IL-2 cytokines in M-CoV-S immunized with AddaVaxTM estimated to be 240.8 pg/ml, 160.4 pg/ml, and 122.2 pg/ml, respectively, whereas the serum concentration of IL-5, IL-4, IL-17a, and IL-10 cytokines appeared to be 68.4 pg/ml, 48.5 pg/ml, 55.4 pg/ml, and 61.8 pg/ml, respectively. In the M-CoV-S and Imject immunized group the peak serum concentration was shown only for IFN-γ, IL-2, and IL-10 cytokines and the titers were 151.4 pg/ml, 92.7 pg/ml, and 46.1 pg/ml, respectively. The serum cytokine profiling of the immunized mice suggests induction of strong Th1 polarized immune responses in AddaVaxTM adjuvanted animals, and the elicitation of IFN-γ and TNF-α also indicates the significant contribution in induction of humoral and cellular responses, however a balanced Th1/Th2 responses as compared to Imject adjuvanted immunized sera.
Molecular modelling and docking of M-CoV-S protein
Homology modeling was used to generate model of M-CoV-S protein of the given sequence using PDB-ID: 5X5C prefusion MERS spike conformation. The Ramachandran plot (Figure S3b) indicates the quality of the modeled protein (Figure 5). The superimposition of the modeled protein with respect to template was 0.4 Å, further indicating that other than loops the major structural portion of the model is overlaid nicely, therefore, we proceed for molecular docking study. From the assessed docking results, the best poses were selected based on their cluster size, with a total of nine clusters considered. Interacting residues were analyzed and depicted in the corresponding figure (Figure 5a). In molecular docking the energetically favorable pose was chosen to compare with co-crystal and for qualitative analysis. One of the limitations in this study is unavailability of the MERS live or pseudovirus to evaluate the neutralizing potential of the immunized sera against the M-CoV-S protein. Hence, we utilized the energy minimization using molecular dynamics simulation study to assessed the binding of MCA1 neutralizing antibody with M-CoV-S protein.18 The binding of MCA1 mAb found to be in the similar binding area as it has been reported in PDB-ID: 6GMQ (interacting with RBD), the paratope residues are common such as Tyr31, Ile49, Ile51, Leu52, Gly98, Asp99, Tyr100, Tyr101, Asp102 and Arg103. Furthermore, the type of interactions was mapped in which several common paratope residues are involved in establishing the similar type of interactions such as hydrogen bonds, salt-bridges and non-bonded interactions (Figure S3c and S3d). The hydrogen bonds were formed between Asp99 (paratop)@Arg525 (epitope), Tyr101@Arg525, Tyr100@Arg525, Asn56@Glu519, Arg103@Glu519 and Asp102@Trp518. The salt-bridges were formed between Asp99@Arg525, Arg47@Asp522, Arg103@Asp522, and Arg103@Glu519. Furthermore, the non-bonded contacts were noticed between Asp99@Trp536, Asp99@Arg525, Tyr100@Tyr523, Tyr100@Tyr524, Arg103@Trp518 and Asp99@Trp536. From interaction fingerprinting it was clearly visible that not only common paratope residues but also the similar property holding residues of epitopes are similar as well, confirming that the antibody binds tightly with modeled spike protein. Moreover, the thermodynamic quantification was also carried out using MM-GBSA module. The docked structure exhibits a binding affinity of −60.03 kcal/mol, while the co-crystal structure demonstrates a binding affinity of −66.17 kal/mol. The comparable binding free energy corroborate well with quantitative analysis as it matched nicely with reported co-crystal structure (PDB-ID: 6GMQ). Overall, the modeling and docking data indicate that antibody MCA1 binds with suitable pose similar to reported co-crystal structure.
Figure 5.
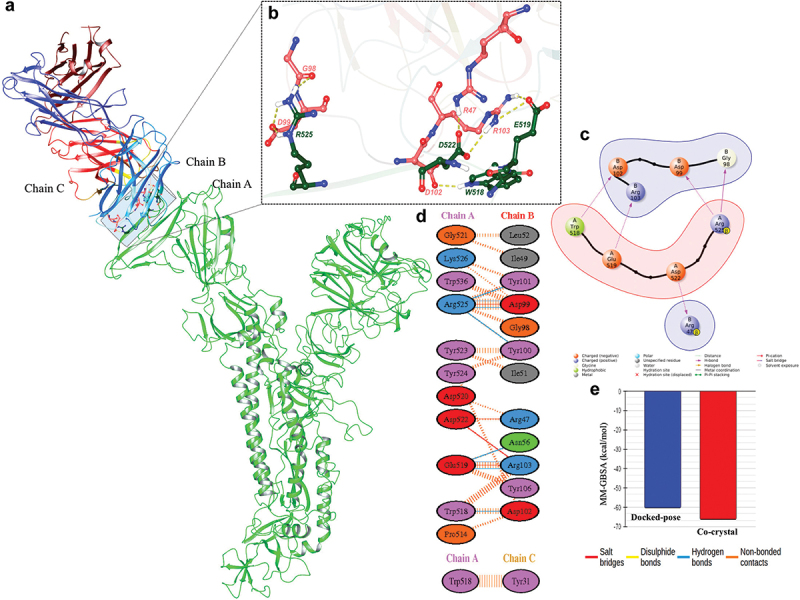
Molecular modeling indicates the possible binding site of antibody: (a) modelled protein (chain A: in green and rendered in cartoon) and antibody (chain B and C: multi-colored and rendered in cartoon). (b) Residues of MCA1 antibody involved in interaction with modeled MERS spike protein. Pink residues represent the MCA1 antibody, and green residues represent the MERS spike protein. All the residues are shown in atom-wise (C: light red/green, N: blue, O: red and H: white). (c) 2D representation of interacting residues. (d) Quantitative interaction map of interacting residues. The color of bubbles indicates the residues basic: blue, acidic: red, aromatic: purple, nonpolar: gray and polar: green. (e) Binding affinity comparison of MCA1antibody with M-CoV-S protein. The modeled in blue and co-crystal in red. All the values are in kcal/mol.
The MERS and other Corona viruses or enveloped viruses like Influenza and HIV-1 contain the N glycosylation sites and are highly glycosylated. Previous studies were shown the sialic acid present in the virus envelope protein N glycans are cross-reactive.37–39 In order to confirm the cross binding due to N-glycans, N-Glycans present in M-CoV-S protein was removed after de-glycosylation with PNGase-F enzyme as described in Materials and Methods section. We found shifting in the molecular weight upon de-glycosylation but the serum specific to SARS-CoV-1-RBD (Supplementary Fig. S4a, Lane 6), SARS-CoV-2-Spike (Supplementary Fig. S4a, Lane 8) and Inf A/Guangdong-Maonan/SWL 1536/2019 (Supplementary Fig. S4a, Lane 11) could still recognized the PNGase-F treated M-CoV-S protein. In order to rule out the nonspecific binding toward mouse polyclonal serum, the M-CoV-S protein was probed with anti-mouse serum collected from normal mice. Protein showed no affinity toward normal mouse serum (Supplementary Fig. S4b, Lane 10). Suggesting the cross binding is not specific only to N-Glycans, but to the existence of some similarity of overlapping sequences of the three viruses’ glycoproteins. However, further studies are needed to confirm the cross reactiveness. In another experiment, BHK21 cells were co-transfected with a plasmid containing a CoV-1 full-length spike and ACE2. Transfection resulted in BHK-21 cells expressing CoV-1 spike over the surface which were incubated with M-CoV-S sera and stained with Alexa 488 dye. The sera showed binding to the CoV-1 spike in the immunofluorescence assay (Supplementary Fig. S4c, e). Similar, cross reactivity also seen in binding of M-CoV-S polyclonal serum with SARS-CoV-2 ancestral Wuhan-Hu-1 pseudo virus infected Vero E6 cells (Supplementary Fig. S4c, e). Further, MDCK-London cells infected with A/Guangdong-Maonan/SWL1536/2019 (H1N1) virus also showed the cross reactivity by binding with M-CoV-S polyclonal sera. Binding was as similar to the polyclonal serum of A/Guangdong-Maonan/SWL 1536/2019 taken as a positive control (Supplementary Fig S4f).
Discussion
The structural spike protein is the major target for vaccine design in Coronaviruses.8 Different platforms based on the spike protein have been used for the development of MERS vaccine candidates.40 Adenovirus ChAdOx1541 or modified vaccinia Ankara [MVA] vector vaccines13 expressing the spike protein were able to induce desirable antibody responses. Tai Wanbo and coworkers showed elicitation of potent neutralizing antibodies using mRNA vaccine platform expressing the monomeric MERS-CoV-RBD protein.42 Even though, MERS-CoV monomeric RBD or spike protein were able to generate desirable protecting immune responses, other studies with other viruses have shown that trimeric native like oligomeric envelope proteins may induce long lasting immune responses.43,44 Recently, Chang Chi-Chieh et.al have expressed the MERS-CoV-S1 ectodomain trimeric protein in the recombinant baculovirus expression system and showed in the presence of QS-21 adjuvant, that the S1 trimeric proteins were able to elicit a strong and enduring memory T cell response.45
The major aim of these studies were three folds; a) to express the pre-fusion trimeric spike soluble recombinant protein antigens in a mammalian expression system which will mimic the spike structure present on the virion surface; b) to expand our knowledge and understanding of spike soluble protein inherent structural, biochemical and biophysical properties; and c) to assess the immunogenicity of the adjuvanted trimeric spike proteins. In the present study, we attempted to express and purify the MERS-CoV trimeric prefusion spike protein using the methodology as described by Pallesen Jesper and coworkers,19 and characterized the biochemical and biophysical properties of purified trimeric oligomers and further evaluated their immunogenicity in presence of AddaVaxTM or Imject alum adjuvants.
The M-CoV-S immunogen was transiently expressed in Expi293F cells and purified to homogeneity via His-tag Ni-NTA affinity chromatography. SDS-PAGE showed a molecular weight of ~ 180 kDa whereas native PAGE showed ~ 1045 kDa thus suggesting formation of higher order oligomers (Figure 1). The homogeneity of the expressed and purified M-CoV-S was confirmed by DLS, which showed a PDI value of 0.265; the range varies from 0 value reflecting the uniform particle size to 1 representing random particle size. Here, the spike trimer proteins size ranges from 33.96 ± 1.20 nm, suggesting higher order structures and some degree of aggregation (Figure 2b), however the proteins showed reasonable colloidal stability as measured by zeta potential (Figure 2b). Furthermore, the maximum fluorescence intensity of M-CoV-S suggested folded pre-fusion spike conformation (Figure 3). The comprehensive biophysical characterization of M-CoV-S protein revealed properly folded, stable recombinant soluble proteins. The M-CoV-S protein resisted temperature induced structural changes and differential scanning colorimetric showed a melting temperature of about 58°C, a desirable characteristic of thermal stability for soluble protein-based immunogens. Additionally, the protein was found to be conformational stable up to 7 days at 37°C (Figure 3), a crucial criterion for vaccine storage in low- and middle-income nations (LMICs) in order to boost immunization rates.
The M-CoV-S immunogen was able to induce robust humoral responses irrespective of the type of adjuvant. In this study, we have evaluated two types of adjuvants, one is the MF-59 squalene-based adjuvant AddavaxTM and the other was the alum-based adjuvant Imject. Mice immunized with M-CoV-S protein induced high IgG titers and pro-inflammatory cytokines to facilitate desirable T cell responses (Figure 4). Notably, M-CoV-S + AddavaxTM immunized mice greatly promoted IgG2a, as compared to the M-CoV-S + Imject, Addavax and Imject alone groups. IgG2a is known to provide antiviral immunity via the influence of cytokines produced by T helper cell 1 (Th1 cell).46 The elevated level of IgG2a in a mouse model is considered significant for MERS coronavirus vaccine efficacy in association with the Th1 subset of CD4+ T cells secreting IFN-γ and IL-12.47 The spike trimers were able to induced potent antibody responses even after single immunization, although the higher antibody responses were shown in spike with AddaVaxTM immunized group. After the boosting the antibody responses were shown to be more or less similar in both the adjuvants immunized group. However, the immunized group with alum showed a higher IgG3 responses as compared to AddaVaxTM immunized group. The mice immune responses were dominated toward Th1 immune responses with production of high IgG2a antibodies, in case of AddaVaxTM adjuvanted immunized mice. It has been documented that the MERS-CoV infection is strongly associated with the downregulation of Th1 and Th2 response, high expression of inflammatory cytokines, and high viral load may contribute to lung inflammation, severe infection, the evolution of pneumonia and Acute respiratory distress syndrome (ARDS) and a higher case fatality rate.48 Consequently, the immunological resistance of a host to viral infections may be strongly influenced by cytokines such as IFN-γ, TNF-α, and IL-2.49,50 The cytokines like IFN-γ and TNF-α have been shown to exert their antiviral activities through several direct (cellular resistance to infection, inhibition to replication, and apoptosis) and indirect mechanisms (up-regulation of major histocompatibility complex class I and class II molecules, activation of APC, and the induction of Th1-type T-cell responses, which are generally associated with protection from viral infections). It has been demonstrated that pre-fused MERS-CoV Spike immunization along with adjuvants prompted a proficient Th1/Th2 response in BALB/c mice, thus it may be considered as a potential immunogen while designing a vaccine against the MERS-CoV.48
To the best of our knowledge, we report for the first time the thermal melting temperature, improved thermal stability, and existence of a pre-fusion spike as a dimer of trimers. The effect of different temperatures on fluorescence intensity and circular dichroism shows the robustness of the structure. The immunogenicity data showed that in the presence of both squalene-based adjuvant Addavax and alum-based adjuvant Imject, the spike oligomers were able to induce high antibody responses. We believe in the absence of licensed vaccines the study adds to the scientific literature and provides promise for future native-like spike-based vaccine designing and development efforts.
The major limitation of this study is the inability to show the neutralization titers of the M-CoV-S immunized sera against the MERS whole virus, due to the unavailability of the MERS live or pseudo viruses at our end. However, the in silico molecular simulation study with M-CoV- S protein with the previously known neutralizing antibody MCA1 (Figure 5) indicated a strong interaction of antibody with spike domain, suggesting that the M-CoV-S trimers have the antigenic properties to induce potential neutralizing antibodies which could effectively block the virus entry and subsequent infection. The binding of MCA1 mAb was found to be in the same binding region as that which PDB-ID: 5GMQ has reported and as shown in our result, which further suggests that the M-CoV-S molecular structure retains the neutralizing epitope site. The MCA1 antibody, which has been isolated from human, has been shown to bind to the MERS-CoV S glycoprotein’s receptor-binding region and prevent the virus from interacting with the human cellular receptor dipeptidyl peptidase 4 (DPP4).18 It has been further shown that the monoclonal antibody totally prevents MERS replication in an in vivo animal model of common marmosets. The presence of this neutralizing binding site or epitope in M-CoV-S thus indicates M-CoV-S trimer proteins might be able to elicit neutralizing antibodies similar to MCA-1 and thus prevent MERS replication. These reverse structure-based studies revealed some fairly desirable properties of the MERS spike trimeric vaccine candidate, in addition to offering a new degree of insight into possibly neutralizing epitopes. Collectively, our results comprehensively characterized the M-CoV-S trimeric proteins physiochemical and immunogenic properties, which may further enhance our knowledge in MERS spike-based vaccine development.
Supplementary Material
Acknowledgments
We sincerely thank Prof Pramod Kumar Garg former Director, THSTI, and Dr. Jayanta Bhattacharya, Dean for full support and valuable input and guidance. We thank our industry partner Panacea Biotec ltd, India and The Coalition for Epidemic Preparedness Innovations (CEPI) scientific coordinators for continuous support and technical guidance. We thank Vikas Maithil, Anup Singh and Amit Kumar from our lab for helping in biochemical and animal experimentation. We thank Prof. Amit Awasthi for timely suggestions and critical inputs. The following reagent was deposited by the Centres for Disease Control and Prevention and obtained through BEI Resources, NIAID, NIH: SARS Related Coronavirus 2, Isolate USA-WA1/2020, NR-52281. We thank Dr. B Graham (VRC/NIAID/NIH) for providing us the spike construct (SARS-2-CoV S 2P) to raise mouse polyclonal sera. We thank the THSTI EAF facility and staffs for providing support and timely help for immunization experiments. We thank Mr. Vijay Kumar of Regional Centre for Biotechnology (RCB), for assisting with DSC and CD experiments. Part of the work was carried out at the Advanced Technology Platform Centre (ATPC) of RCB.
Funding Statement
In this study the MERS antigen design and characterization was partially supported by the grant from the Coalition for Epidemic Preparedness Innovations (“CEPI”) towards a Consortium involving THSTI and Panacea Biotec ltd., to develop a Multi-epitope, Nanoparticle based broadly protective Beta coronavirus candidate vaccine (“Project”). The other works were supported by Translational Health Science and Technology Institute (THSTI) core funding and INDCEPI project, an institute functioning under the Biotechnology Research and Innovation Council (BRIC) of the Department of Biotechnology, Ministry of Science and Technology, Government of India.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Author contribution
S.S and S.A. designed the immunogen; S.R. expressed and purified the immunogen under the guidance of S.A. P.V, R.A, V.K carried out the biochemical and biophysical characterization. P.V. and Sh.S. (Shikha Saxena) carried out in vivo study, A.K. assisted in in vivo experiments for animal handling during immunization and blood collection at different time points. R.K. and B.L conducted the CoV-1 and CoV-2 in vitro studies, Sh.S carried out in vitro experiment with Influenza. Sh.A. (Shailendra Asthana) and G.S. made the bioinformatics analysis and molecular modelling. S.S. and S.A conceived the study and designed experiments. S.S. S.A. S.R., P.V., R.A, V.K, Sh.A, Sh.S and M.S. analyzed the data. S.S. wrote the original manuscript. S.A. S.R., P.V., R.A, V.K, Sh.A, Sh.S and M.S edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2024.2351664.
Data availability
All is available from corresponding author upon request.
Abbreviations
SARS-CoV: severe acute respiratory syndrome coronavirus, MERS-CoV: Middle East Respiratory Syndrome, M-CoV-S: Middle East Respiratory Syndrome Coronavirus Spike, DPP4: dipeptidyl peptidase-4, TLR: Toll-like Receptor, RBD: Receptor binding domain, DNA: deoxynucleic acid
References
- 1.Behl A, Nair A, Mohagaonkar S, Yadav P, Gambhir K, Tyagi N, Sharma RK, Butola BS, Sharma N.. Threat, challenges, and preparedness for future pandemics: a descriptive review of phylogenetic analysis based predictions. Infect Genet Evol [Internet]. 2022;98:105217. [cited 2023 Dec 6]. Available from. https://pubmed.ncbi.nlm.nih.gov/35065303/. [DOI] [PubMed] [Google Scholar]
- 2.Zaki AM, van Boheemen S, Bestebroer TM, ADME O, Fouchier RAM. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med [Internet]. 2012;367(19):1814–14Available from.cited 2023 Dec 6. https://pubmed.ncbi.nlm.nih.gov/23075143/. [DOI] [PubMed] [Google Scholar]
- 3.Azhar EI, Hui DSC, Memish ZA, Drosten C, Zumla A. The middle east respiratory syndrome (MERS). Infect Dis Clin North Am [Internet]. 2019;33(4):891–905Available from.cited 2023 Dec 6. https://pubmed.ncbi.nlm.nih.gov/31668197/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ki M. MERS outbreak in Korea: hospital-to-hospital transmission. Epidemiol Health [Internet]. 2015;37:e2015033. [cited 2023 Dec 6]. Available from. https://pubmed.ncbi.nlm.nih.gov/26212508/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO . MERS SITUATION UPDATE February 2023 GLOBAL and REGIONAL CASES. 2023.
- 6.Tai W, Zhang X, Yang Y, Zhu J, Du L. Advances in mRNA and other vaccines against MERS-CoV. Transl Res [Internet]. 2022;242:20–37. [cited 2023 Dec 6]. Available from. https://pubmed.ncbi.nlm.nih.gov/34801748/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang N, Shi X, Jiang L, Zhang S, Wang D, Tong P, Guo D, Fu L, Cui Y, Liu X. et al. Structure of MERS-CoV spike receptor-binding domain complexed with human receptor DPP4. Cell Res [Internet] [Available from]. 2013;23(8):986–93. [cited 2023 Dec 6]. doi: 10.1038/cr.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Du L, Yang Y, Zhou Y, Lu L, Li F, Jiang S. MERS-CoV spike protein: a key target for antivirals. Expert Opin Ther Targets [Internet]. 2017;21(2):131–43Available from.cited 2023 Dec 6. https://pubmed.ncbi.nlm.nih.gov/27936982/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yuan Y, Cao D, Zhang Y, Ma J, Qi J, Wang Q, Lu G, Wu Y, Yan J, Shi Y. et al. Cryo-EM structures of MERS-CoV and SARS-CoV spike glycoproteins reveal the dynamic receptor binding domains. Nat Commun [Internet]. 2017;8(1): [cited 2023 Dec 6]. Available from. doi: 10.1038/ncomms15092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beniac DR, SL DV, Andonov A, He R, Booth TF. Conformational reorganization of the SARS coronavirus spike following receptor binding: implications for membrane fusion. PLoS One [Internet]. 2007;2(10):e1082.Available from.cited 2023 Dec 6. https://pubmed.ncbi.nlm.nih.gov/17957264/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bosch BJ, van der Zee R, CAM DH, Rottier PJM. The coronavirus spike protein is a class I virus fusion protein: structural and functional characterization of the fusion core complex. J Virol [Internet]. 2003;77(16):8801–11Available from.cited 2023 Dec 6. https://pubmed.ncbi.nlm.nih.gov/12885899/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Modjarrad K, Roberts CC, Mills KT, Castellano AR, Paolino K, Muthumani K, Reuschel EL, Robb ML, Racine T, Don OM. et al. Safety and immunogenicity of an anti-middle east respiratory syndrome coronavirus DNA vaccine: a phase 1, open-label, single-arm, dose-escalation trial. Lancet Infect Dis [Internet]. 2019;19(9):1013–22. cited 2023 Dec 6. Available from. https://pubmed.ncbi.nlm.nih.gov/31351922/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koch T, Dahlke C, Fathi A, Kupke A, Krähling V, Okba NMA, Halwe S, Rohde C, Eickmann M, Volz A. et al. Safety and immunogenicity of a modified vaccinia virus Ankara vector vaccine candidate for middle east respiratory syndrome: an open-label, phase 1 trial. Lancet infect dis [internet]. 2020;20(7):827–38. cited 2023 Dec 6. Available from. https://pubmed.ncbi.nlm.nih.gov/32325037/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Folegatti PM, Bittaye M, Flaxman A, Lopez FR, Bellamy D, Kupke A, Mair C, Makinson R, Sheridan J, Rohde C. et al. Safety and immunogenicity of a candidate middle east respiratory syndrome coronavirus viral-vectored vaccine: a dose-escalation, open-label, non-randomised, uncontrolled, phase 1 trial. Lancet Infect Dis [Internet]. 2020;20(7):816–26. cited 2023 Dec 6. Available from. https://pubmed.ncbi.nlm.nih.gov/32325038/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu L, Wei Q, Lin Q, Fang J, Wang H, Kwok H, Tang H, Nishiura K, Peng J, Tan Z. et al.Anti–spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection JCI Insight [Internet] 2019cited 2023 Dec 154Available fromhttps://pmc/articles/PMC6478436/4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou P, Li Z, Xie L, An D, Fan Y, Wang X, Li Y, Liu X, Wu J, Li G. et al. Research progress and challenges to coronavirus vaccine development. J Med Virol [Internet]. 2021;93(2):741–54. cited 2023 Dec 6. Available from. https://pubmed.ncbi.nlm.nih.gov/32936465/. [DOI] [PubMed] [Google Scholar]
- 17.Bigay J, Le Grand R, Martinon F, Maisonnasse P. Vaccine-associated enhanced disease in humans and animal models: lessons and challenges for vaccine development. Front Microbiol [Internet] Available from. 2022;13 [cited 2023 Dec 6]. https://pubmed.ncbi.nlm.nih.gov/36033843/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Z, Bao L, Chen C, Zou T, Xue Y, Li F, Lv Q, Gu S, Gao X, Cui S. et al. Human neutralizing monoclonal antibody inhibition of middle east respiratory syndrome coronavirus replication in the common marmoset. J Infect Dis [internet]. 2017;215(12):1807–15. cited 2023 Dec 9. Available from. https://pubmed.ncbi.nlm.nih.gov/28472421/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pallesen J, Wang N, Corbett KS, Wrapp D, Kirchdoerfer RN, Turner HL, Cottrell CA, Becker MM, Wang L, Shi W. et al. Immunogenicity and structures of a rationally designed prefusion MERS-CoV spike antigen. Proc Natl Acad Sci U S A [InternetAvailable from]. 2017;114(35):E7348–57. [cited 2023 Dec 6]. doi: 10.1073/pnas.1707304114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Çelik İ, Saatçi E, Eyüboğlu FÖ. Emerging and reemerging respiratory viral infections up to covid-19. Turkish J Med Sci [Internet]. 2020;50(SI–1):557–62Available from.cited 2023 Dec 6. https://pubmed.ncbi.nlm.nih.gov/32293833/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang TJ, Yu PY, Chang YC, Hsu S Te D. D614G mutation in the SARS-CoV-2 spike protein enhances viral fitness by desensitizing it to temperature-dependent denaturation. J Biol Chem. 2021;297(4):101238.Available from.cited 2023 Dec 6. https://pubmed.ncbi.nlm.nih.gov/34563540/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joshi A, Akhtar N, Sharma NR, Kaushik V, Borkotoky S. MERS virus spike protein HTL-epitopes selection and multi-epitope vaccine design using computational biology. J Biomol Struct Dyn [Internet]. cited 2023 Dec 6]; Available from 2023;41(22):12464–79. doi: 10.1080/07391102.2023.2191137. [DOI] [PubMed] [Google Scholar]
- 23.Zhou S, Zou H, Liu C, Zang M, Liu T. Combining deep neural networks for protein secondary structure prediction. IEEE Access. 2020;8:84362–70. doi: 10.1109/ACCESS.2020.2992084. [DOI] [Google Scholar]
- 24.Walls AC, Xiong X, Park YJ, Tortorici MA, Snijder J, Quispe J, Cameroni E, Gopal R, Dai M, Lanzavecchia A. et al. Unexpected receptor functional mimicry elucidates activation of coronavirus fusion. Cell [Internet] [Available from]. 2019;176(5):1026–39.e15. [cited 2023 Dec 9]. doi: 10.1016/j.cell.2018.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Srivastava M, Mittal L, Kumari A, Agrahari AK, Singh M, Mathur R, Asthana S. Characterizing (un)binding mechanism of USP7 inhibitors to unravel the cause of enhanced binding potencies at allosteric checkpoint. Protein Sci [Internet] [Available from]. 2022. [cited 2023 Dec 9];31(9). https://pubmed.ncbi.nlm.nih.gov/36629250/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumari A, Mittal L, Srivastava M, Pathak DP, Asthana S. Deciphering the structural determinants critical in attaining the FXR partial agonism. J Phys Chem B InternetAvailable from. 2023;127(2):465–85. [cited 2023 Dec 9]. doi: 10.1021/acs.jpcb.2c06325. [DOI] [PubMed] [Google Scholar]
- 27.Srivastava M, Mittal L, Sarmadhikari D, Singh VK, Fais A, Kumar A, Asthana S. Template entrance channel as possible allosteric inhibition and resistance site for quinolines tricyclic derivatives in RNA dependent RNA polymerase of bovine viral diarrhea virus. Pharmaceuticals (Basel) InternetAvailable from. 2023;16(3):376. [cited 2023 Dec 9]. doi: 10.3390/ph16030376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mittal L, Tonk RK, Awasthi A, Asthana S. Harnessing the druggability at orthosteric and allosteric sites of PD-1 for small molecule discovery by an integrated in silico pipeline. Comput Biol Chem [Internet]. 2023;107:107965. [cited 2023 Dec 9]. Available from. https://pubmed.ncbi.nlm.nih.gov/37826990/. [DOI] [PubMed] [Google Scholar]
- 29.Walls AC, Tortorici MA, Frenz B, Snijder J, Li W, Rey FA, DiMaio F, Bosch BJ, Veesler D. Glycan shield and epitope masking of a coronavirus spike protein observed by cryo-electron microscopy. Nat Struct Mol Biol [Internet] Available from. 2016;23(10):899–905. [cited 2023 Dec 6]. doi: 10.1038/nsmb.3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pandey VK, Sharma R, Prajapati GK, Mohanta TK, Mishra AK. N-glycosylation, a leading role in viral infection and immunity development. Mol Biol Rep [Internet] Available from. 2022;49(8):8109–20. [cited 2023 Dec 6]. doi: 10.1007/s11033-022-07359-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arruda HRS, Lima TM, Alvim RGF, Victorio FBA, Abreu DPB, Marsili FF, Cruz KD, Marques MA, Sosa-Acosta P, Quinones-Vega M. et al. Conformational stability of SARS-CoV-2 glycoprotein spike variants. iSci [Available from]. 2023;26(1):105696. [cited 2024 Mar 18]. doi: 10.1016/j.isci.2022.105696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hauser A, Carnell G, Held K, Sulbaran G, Tischbierek N, Rogers L, Pollakis G, Tonks P, Hoelscher M, Ding S. et al. Stepwise conformational stabilization of a HIV-1 clade C consensus envelope trimer immunogen impacts the profile of vaccine-induced antibody responses. Vaccines [Internet] [Available from]. 2021;9(7):750. [cited 2024 Mar 18]. doi: 10.3390/vaccines9070750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Juraszek J, Rutten L, Blokland S, Bouchier P, Voorzaat R, Ritschel T, Bakkers MJG, Renault LLR. Langedijk JPM. Stabilizing the closed SARS-CoV-2 spike trimer. Nat Commun 2021 121 [Internet] Available from2021cited 2024 Mar 18121–8https://www.nature.com/articles/s41467-020-20321-x1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao T, Cai Y, Jiang Y, He X, Wei Y, Yu Y, Tian X. Vaccine adjuvants: mechanisms and platforms. Signal Transduct Target Ther [Internet] [Available from]. 2023. [cited 2023 Dec 6];8(1). https://pubmed.ncbi.nlm.nih.gov/37468460/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akache B, Stark FC, Agbayani G, Renner TM, MJ M. Adjuvants: engineering protective immune responses in human and veterinary vaccines. Methods Mol Biol [Internet]. 2022;2412:179–231. [cited 2023 Dec 6]. Available from. https://pubmed.ncbi.nlm.nih.gov/34918246/. [DOI] [PubMed] [Google Scholar]
- 36.Awate S, Babiuk LA, Mutwiri G. Mechanisms of action of adjuvants. Front Immunol [Internet] Available from. 2013;4 [cited 2023 Dec 6]. https://pubmed.ncbi.nlm.nih.gov/23720661/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perween R, PraveenKumar M, Shrivastava T, Parray HA, Singh V, Singh S, Chiranjivi A, Jakhar K, Sonar S, Tiwari M. et al. The SARS CoV-2 spike directed non-neutralizing polyclonal antibodies cross-react with Human immunodeficiency virus (HIV-1) gp41. Int Immunopharmacol [Internet]. 2021. cited 2023 Dec 6. 101. 108187. Available from. https://pubmed.ncbi.nlm.nih.gov/34649114/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mishra N, Kumar S, Singh S, Bansal T, Jain N, Saluja S, Kumar R, Bhattacharyya S, Palanichamy JK, Mir RA. et al. Cross-neutralization of SARS-CoV-2 by HIV-1 specific broadly neutralizing antibodies and polyclonal plasma. PLOS Pathog [Internet] [Available from]. 2021;17(9):e1009958. [cited 2023 Dec 6]. doi: 10.1371/journal.ppat.1009958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lapidus S, Liu F, Casanovas-Massana A, Dai Y, Huck JD, Lucas C, Klein J, Filler RB, Strine MS, Sy M. et al. Plasmodium infection is associated with cross-reactive antibodies to carbohydrate epitopes on the SARS-CoV-2 spike protein. Sci Rep [Internet]. 2022;12(1): [cited 2023 Dec 6]. Available from. doi: 10.1038/s41598-022-26709-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bosaeed M, Alharbi NK. Vaccination strategies for mitigation of MERS-CoV outbreaks. Lancet Glob Heal [Internet]. 2023;11(5):e644–5Available from.cited 2023 Dec 6. https://pubmed.ncbi.nlm.nih.gov/37061302/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bosaeed M, Balkhy HH, Almaziad S, Aljami HA, Alhatmi H, Alanazi H, Alahmadi M, Jawhary A, Alenazi MW, Almasoud A. et al. Safety and immunogenicity of ChAdOx1 MERS vaccine candidate in healthy middle eastern adults (MERS002): an open-label, non-randomised, dose-escalation, phase 1b trial. The Lancet Microbe [Internet] [Available from]. 2022;3(1):e11–20. [cited 2023 Dec 6]. doi: 10.1016/S2666-5247(21)00193-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tai W, Zheng J, Zhang X, Shi J, Wang G, Guan X, Zhu J, Perlman S, Du L. MERS-CoV RBD-mRNA vaccine induces potent and broadly neutralizing antibodies with protection against MERS-CoV infection. Virus Res [Internet]. 2023;334:199156. [cited 2023 Dec 6]. Available from. https://pubmed.ncbi.nlm.nih.gov/37336390/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tjärnhage E, Brown D, Bogen B, Andersen TK, Grødeland GG, Subbarao K. Trimeric, APC-Targeted subunit vaccines protect mice against seasonal and pandemic influenza. J Virol [Internet] Available from. 2023;97(2). [cited 2023 Dec 6]. doi: 10.1128/jvi.01694-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Y, Dai L, Feng X, Gao R, Zhang N, Wang B, Han J, Zou Q, Guo X, Zhu H. et al. Fast and long-lasting immune response to S-trimer COVID-19 vaccine adjuvanted by PIKA. Mol Biomed [internet]. 2021;2(1): [cited 2023 Dec 6]. Available from. doi: 10.1186/s43556-021-00054-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang CC, Algaissi A, Lai CC, Chang CK, Lin JS, Wang YS, Chang BH, Chang YC, Chen WT, Fan YQ. et al. Subunit vaccines with a saponin-based adjuvant boost humoral and cellular immunity to MERS coronavirus. Vaccine [Available from]. 2023;41(21):3337–46. [cited 2023 Dec 6]. doi: 10.1016/j.vaccine.2023.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coutelier JP, JTM VDL, Heessen FWA, Warnier G, Van Snick J. IgG2a restriction of murine antibodies elicited by viral infections. J Exp Med [Internet]. 1987;165(1):64.Available from.cited 2023 Dec 8. https://pmc/articles/PMC2188250/?report=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Faure E, Poissy J, Goffard A, Fournier C, Kipnis E, Titecat M, Bortolotti P, Martinez L, Dubucquoi S, Dessein R. et al. Distinct immune response in two MERS-CoV-infected patients: can we go from bench to bedside? PLoS one [internet]. 2014;9(2):e88716. cited 2023 Dec 8. Available from. https://pmc/articles/PMC3925152/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alosaimi B, Hamed ME, Naeem A, Alsharef AA, AlQahtani SY, AlDosari KM, Alamri AA, Al-Eisa K, Khojah T, Assiri AM. et al. MERS-CoV infection is associated with downregulation of genes encoding Th1 and Th2 cytokines/chemokines and elevated inflammatory innate immune response in the lower respiratory tract. Cytokine [Internet]. [Available from]. 2020. [cited 2023 Dec 6];126:154895. doi: 10.1016/j.cyto.2019.154895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johnston SC, Johnson JC, Stonier SW, Lin KL, Kisalu NK, Hensley LE, Rimoin AW. Cytokine modulation correlates with severity of monkeypox disease in humans. J Clin Virol [Internet]. 2015;63:42–5. [cited 2023 Dec 6]. Available from. https://pubmed.ncbi.nlm.nih.gov/25600603/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dittmer U, Peterson KE, Messer R, Stromnes IM, Race B, Hasenkrug KJ. Role of interleukin-4 (IL-4), IL-12, and gamma interferon in primary and vaccine-primed immune responses to friend retrovirus infection. J Virol [Internet] Available from. 2001;75(2):654–60. [cited 2023 Dec 6]. doi: 10.1128/JVI.75.2.654-660.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All is available from corresponding author upon request.


