Abstract
Ovarian cancer (OC) is the most lethal gynecological tumor, characterized by its insidious and frequently recurring metastatic progression. Owing to limited early screening methods, over 70% of OC cases are diagnosed at advanced stages, typically stage III or IV. Recently, N6-methyladenosine (m6A) modification has emerged as a hotspot of epigenetic research, representing a significant endogenous RNA modification in higher eukaryotes. Numerous studies have reported that m6A-related regulatory factors play pivotal roles in tumor development through diverse mechanisms. Moreover, recent studies have indicated the aberrant expression of multiple regulatory factors in OC. Therefore, this paper comprehensively reviews research advancements concerning m6A in OC, aiming to elucidate the regulatory mechanism of m6A-associated regulators on pivotal aspects, such as proliferation, invasion, metastasis, and drug resistance, in OC. Furthermore, it discusses the potential of m6A-associated regulators as early diagnostic markers and therapeutic targets, thus contributing to the diagnosis and treatment of OC.
Keywords: N6-methyladenosine (m6A), ovarian cancer, tumor microenvironment, prognosis, diagnosis, treatment, metastatic progression, epigenetic research, endogenous RNA modification
Plain Language Summary
Ovarian cancer (OC) presents a formidable challenge in the medical field, often detected at advanced stages, necessitating urgent exploration of diagnostic and therapeutic avenues. This review delves into the intricate role of N6-methyladenosine (m6A) RNA modification in OC, a dynamic epigenetic process increasingly recognized for its regulatory role in cancer biology. Highlighting recent advancements, the review sheds light on how m6A-related factors influence crucial aspects of OC progression, including tumor growth, metastasis, and resistance to treatment. Specifically, m6A methyltransferases, binding proteins, and demethylases exert multifaceted effects on OC progression, influencing the expression of pivotal oncogenes and tumor suppressors. While promising, translating these insights into effective therapies requires further investigation. By comprehensively understanding the influence of m6A on OC, there lies hope for developing improved diagnostic techniques and novel treatment strategies to combat this complex disease.
Introduction
Ovarian cancer (OC) develops insidiously, with inconspicuous early symptoms, limited early screening methods, and a high propensity for recurrence and metastasis. Alarmingly, over 70% of OC cases remain undetected until they have progressed to advanced stages, generally stage III or IV. 1 The latest statistics indicate a 5-year survival rate of only 49.7% patients with OC. 2 Therefore, it is imperative to explore potential diagnostics, therapeutic targets, and biomarkers specific to OC by comprehensively studying its tumorigenic mechanisms, which may significantly improve the clinical outcomes of patients with OC.
Recent advancements in RNA epitranscriptomics have spearheaded the search for novel biomarkers, with numerous studies highlighting the significance of epigenetic dysregulation across various diseases, especially tumors. Among RNA modifications, N6-methyladenosine (m6A) stands out as one of the most prominent RNA modifications in the field of epitranscriptomics. While the initial descriptions of m6A date back to the 1970s,3,4 the specific functions and underlying mechanisms have only been elucidated in recent years.5,6 M6A is the most common endogenous methylation modification in eukaryotic RNAs, 7 preferentially occurring on the adenosine nucleotide at the nitrogen-6 position in the consensus motif ‘RRm6ACH’ (where R = G or A; H = A, C, or U).8-10 Since its initial discovery, m6A has been implicated in an extensive array of RNA metabolic processes, including RNA splicing, structural remodeling, nuclear export, translation, stability, and degradation. 11 These biological functions, as well as the dynamic reversible processes of m6A modification, are regulated by 3 proteins: “writers” (methyltransferases), “erasers” (demethylases), and “readers” (m6A-binding proteins).7,12 Owing to its profound impact on RNA metabolism, m6A is involved in various physiological and pathological processes, including immunity, 13 viral infections, 14 inflammation, 15 metabolism, 16 embryogenesis, 17 and cancer. 18 Currently, numerous studies have underscored the close association between m6A and the development and prognosis of malignant tumors. In this paper, we review the latest findings from m6A research in OC, as well as the mechanisms through which m6A regulates the proliferation, migration, invasion, and drug resistance of OC cells, while also summarizing its impact on the development and prognosis of OC.
Ovarian Cancer and “Writers”
M6A “writers” refer to proteins involved in the assembly of the methyltransferase complex that mediates the formation of m6A modifications. Within this complex, methyltransferase-like 3 (METTL3) is the earliest identified and most pivotal component, methyltransferase-like 14 (METTL14) provides structural support. Moreover, Wilms’ tumor 1-associated protein (WTAP) plays a crucial role in targeting the METTL3/14 heterodimer to nuclear speckles. 19 Additionally, RNA-binding motif protein 15/15B (RBM15/15B), vir-like m6A methyltransferase associated (VIRMA), and zinc finger CCCH-type containing 13 (ZC3H13) are implicated in the formation of the methyltransferase complex. The aforementioned methyltransferases exert diverse functions in OC via distinct molecular mechanisms, as summarized in Table 1 and Figure 1.
Table 1.
Roles of Methyltransferase in Ovarian Cancer (OC).
| Writers | Genes Regulated | Mechanisms | Roles in OC | Year | Reference |
|---|---|---|---|---|---|
| METTL3 | AXL | Promotes EMT signaling by enhancing AXL translation | Promotes OC proliferation, migration, and invasion | 2018 | 24 |
| METTL3 | EIF3C, AXL, CSF-1, FZD10 | Upregulates oncogene (EIF3C, AXL, CSF-1, FZD10) expression independently of METTL14 and WTAP | Promotes OC proliferation, migration, and invasion | 2020 | 25 |
| METTL3 | pri-miR-1246 | Upregulates miR-1246 expression via m6A modification and inhibits CCNG2 | Promotes proliferation, migration, and invasion and inhibits apoptosis in OC | 2021 | 26 |
| METTL3 | RHPN1-AS1 | Upregulates the expression of RHPN1-AS1 by enhancing its stability, thereby activates the PI3K/AKT signaling pathway | Enhances cisplatin resistance in OC | 2022 | 27 |
| METTL3 | AKT | METTL3 downregulation inhibits activation of the AKT pathway and its downstream effectors | Promotes proliferation, migration, and invasion and inhibits apoptosis in OC | 2020 | 28 |
| METTL3 | IFFO1 | METTL3 reduces IFFO1 mRNA stability at post-transcriptional level | Promotes metastasis and drug resistance in OC | 2023 | 29 |
| METTL3 | pri-miR-126-5p | Promotes miR-126-5p expression via m6A modification and thereby activates the PTEN/PI3K/Akt/mTOR signaling pathway | Promotes OC proliferation, migration, and invasion | 2020 | 30 |
| METTL3 | BIRC5 | METTL3 and IGF2BP1 co-regulate BIRC5 expression and stability through m6A modification | Promotes cisplatin resistance in OC | 2023 | 31 |
| METTL3 | RIPK4 | METTL3-mediated m6A modification upregulates RIPK4, with YTHDF1 enhancing RIPK4 mRNA stability, activating the NF-κB signaling pathway | Promotes proliferation and cisplatin resistance in OC | 2024 | 32 |
| METTL3 | circPLPP4 | METTL3/IGF2BP1 enhances circPLPP4 stability, promoting its expression to sponge miR-136/PIK3R1 axis to confer CDDP resistance | Promotes cisplatin resistance in OC | 2024 | 33 |
| METTL3 | VGLL1 | METTL3-mediated m6A modification contributes to VGLL1 upregulation in an IGF2BP2 recognition-dependent manner | Promotes proliferation and metastasis in OC | 2024 | 34 |
| METTL3 | MEG3 | METTL3/YTHDF2 facilitates MEG3 degradation and promotes OC proliferation and metastasis via the miR-885-5p/VASH1 axis | Promotes proliferation and metastasis in OC | 2024 | 35 |
| METTL14 | TROAP | Decreases TROAP mRNA stability | Inhibits OC proliferation | 2022 | 43 |
| WTAP | AKT, ERK, JNK, p38 |
Activates AKT and MAPK signaling pathways | Promotes proliferation, migration, and invasion and inhibits apoptosis in OC | 2019 | 47 |
| WTAP | FAM76 A, HBS1L | May contribute to the stability of FAM76 A and HBS1L through a post-transcriptional pathway | Promotes proliferation, migration, and invasion in OC | 2020 | 48 |
| WTAP | miR-200 | Promotes miR-200 maturation to regulate HK2 | Promotes proliferation, migration, and invasion in OC | 2022 | 49 |
| WTAP | EGR3 | Inhibits EGR3 transcription and translation | Promotes proliferation, migration, and invasion and inhibits apoptosis in OC | 2020 | 50 |
| VIRMA | ENO1 | VIRMA is transcriptionally activated by SPI1 to enhance ENO1 mRNA stability in an m6A-dependent pathway | Promotes proliferation, migration, and invasion and inhibits apoptosis in OC | 2023 | 51 |
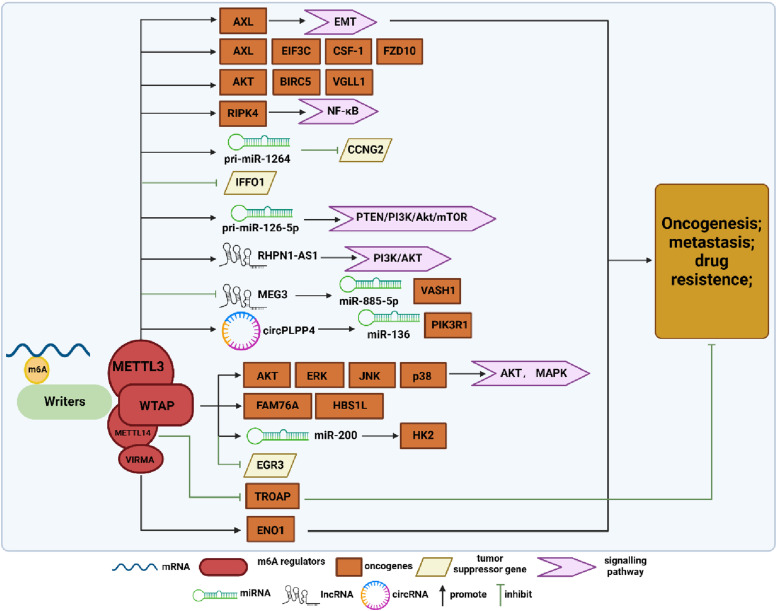
Figure 1.
Summary diagram illustrating m6A methyltransferase regulation in ovarian cancer (OC) development. The methyltransferase complex mediates m6A modification, with METTL3, METTL14, WTAP, and VIRMA independently influence the tumorigenesis, metastasis, and drug resistance of OC. METTL3 promotes OC progression by upregulating the expression of AXL, EIF3C, CSF-1, FZD-10, AKT, BIRC5, VGLL, RIPK4, pri-miR-1264, pri-miR-126-5p, lncRHPN1-AS1, and circPLPP4, while inhibiting the expression of IFFO1 and lncMEG3. WTAP stimulates the malignant biological behavior of OC by upregulating the expression of AKT, ERK, JNK, p38, FAM76A, HBS1L, and miR-200 and inhibiting EGR3 expression. METTL14 and VIRMA respectively act on TROAP and ENO1, exerting inhibitory and promoting effects on OC.
Ovarian cancer and METTL3
METTL3, the most critical component of the m6A methyltransferase complex, is a highly conserved S-adenosylmethionine (SAM)-binding protein. 20 Several studies have underscored the significance of METTL3 in the development and progression of various cancers. 21 In studies related to hepatocellular carcinoma and lung cancer, METTL3 has been identified as an oncogene that promotes tumor proliferation, migration, and colony-forming ability, thereby correlating with poor prognosis in cancer patients.22,23
The expression of METTL3 in OC tissues has been reported to be significantly higher than that in normal ovarian tissues. 24 Additionally, METTL3 has been identified as a regulator of m6A methylation in epithelial ovarian cancer (EOC), independent of METTL14 and WTAP. 25 Regarding the specific mechanism of METTL3 in OC development, various studies have proposed different explanations. For instance, Bi et al demonstrated that METTL3 could recognize the m6A modification site of pri-miR-1246, leading to the upregulation of miR-1246 expression and the inhibition of cyclin G2 (CCNG2), which accelerated the proliferation, migration, and invasion of OC cells while inhibiting their apoptosis, ultimately promoting OC progression. 26 Cui et al demonstrated that in cisplatin-resistant OC cells, METTL3 could enhance the stability of RHPN1-AS1 (lncRNA RHPN1 antisense RNA 1) through m6A modification, 27 which in turn promoted the expression of RHPN1-AS1, activated the PI3K/AKT signaling pathway, and subsequently enhanced cisplatin resistance in OC cells. Additionally, Wenfeng Hua et al reported that METTL3 could indirectly regulate AXL receptor tyrosine kinase (AXL), which plays a pro-oncogenic role in various cancers, including OC. 24 Likewise, Liang et al proposed that METTL3 promotes the malignant progression of OC by activating the AKT-related signaling pathway and thereby stimulating a series of downstream effectors, including p70S6K and Cyclin D1. 28 Moreover, Zhang et al experimentally validated that the low-level expression of oncogene intermediate filament family orphan 1 (IFFO1) in OC was modulated by METTL3/YTHDF2 participation. METTL3, when highly expressed, reduced the post-transcriptional stability of IFFO1, which in turn was degraded by YTHDF2. Consequently, the mechanisms of anti-tumor metastasis and IFFO1-mediated drug resistance reversal were inhibited. 29 Bi et al, conversely, inhibited the development of OC by knocking down METTL3 to suppress the upregulation of phosphatase and tensin homolog (PTEN) mediated by miR-126-5p (a key biomarker for several cancers), thereby preventing the activation of the PI3K/Akt/mTOR pathway. 30 Additionally, Fan et al reported an upregulation of baculoviral IAP repeat containing 5 (BIRC5), an inhibitor of the apoptosis (IAP) family protein, in cisplatin-resistant OC cell lines. They further showed that BIRC5 was upregulated and stabilized by METTL3-mediated m6A modification and IGF2BP1 binding, which in turn promoted platinum resistance in OC. 31 Furthermore, Yin et al demonstrated that the upregulation of receptor-interacting protein kinase 4 (RIPK4), which acts as an oncogene in OC, was facilitated by METTL3-mediated m6A modification. This process was accompanied by concurrent enhancement of RIPK4 mRNA stability mediated by YTHDF1, leading to NF-κB activation and subsequent tumor growth and cisplatin resistance. 32 Moreover, CircPLPP4 was found to be remarkably overexpressed in chemoresistant OC tissues and cells, with its increased levels regulated by METTL3/IGF2BP1 through modulation of its stability. 33 Similarly, vestigial-like 1 (VGLL1) was also found to be upregulated by the combined action of METTL3 and IGF2BP2 in an m6A-dependent manner, which in turn promoted OC proliferation and metastasis. 34 Additionally, a mechanistic study revealed that METTL3/YTHDF2-mediated methylation induces the degradation of the tumor suppressor lncRNA MEG3, which in turn releases the tumor suppression effect and subsequently promotes OC proliferation and metastasis through the miR-885-5p/VASH1 axis. 35 These findings collectively affirm that METTL3 is dysregulated in OC and affects multiple signaling pathways in an m6A-dependent manner, thus playing a pro-cancer role in OC.
Ovarian cancer and METTL14
METTL14 acts as a key component of the m6A methyltransferase complex and influences oncogene expression in tumors by modulating RNA stability and degradation.36-38 Interestingly, in breast cancer cells, METTL14 has been implicated in a pro-tumorigenic role, where it regulates the m6A methylation level of key mRNAs involved in the epithelial-to-mesenchymal transition (EMT). However, its expression is downregulated in various tumors, including hepatocellular carcinoma, bladder cancer, endometrial carcinoma, and glioblastoma, where it functions as a tumor suppressor.39,40 Moreover, in digestive malignant tumors, METTL14 has been observed to play a contradictory role in the regulation of digestive system tumors, 41 with similar findings reported in the studies related to OC. Trophinin-associated protein (TROAP, also known as TASTIN) has been implicated in the proliferation, invasion, and migration processes of various cancers. 42 Li et al demonstrated that METTL14 negatively regulates the expression of TROAP in an m6A-dependent manner, thereby inhibiting OC cell proliferation. 43 However, in another retrospective study, Wei et al proposed a different hypothesis suggesting that high METTL14 expression elevates the level of m6A modification in OC cells, thereby promoting their proliferation, invasion, and migration. 44 Presently, there are limited studies on the association of METTL14 with OC; therefore, further exploration is needed to elucidate the multiple molecular mechanisms underlying the effect of METTL14 on the malignant biological behaviors of OC and the potential theoretical rationale for targeted therapies.
Ovarian cancer and WTAP
WTAP plays a crucial role in localizing the methyltransferases METTL3–METTL14 to nuclear speckles and engages in post-translational regulation, including binding to the 3′-untranslated region (UTR) to enhance mRNA stability and acting as a spliceosome for selective splicing of precursor mRNAs.45,46 Similar to METTL3, WTAP is highly expressed in high-grade serous OC tissues, plays pro-proliferative, migratory, and anti-apoptotic roles, and is associated with patient prognosis and survival. 47 Wang et al confirmed a significant positive correlation between WTAP expression and the expression of Family with sequence similarity 76 member A (FAM76A) and HBS1-like translational GTPase (HBS1L). They hypothesized that WTAP mediates the m6A modification of FAM76A and HBS1L, thereby synergizing with positive transcripts to promote their stability, 48 which in turn promotes OC progression. Additionally, Lyu et al reported that WTAP drives the malignant progression of OC by promoting the maturation of miR-200, which subsequently affects hexokinase 2 (HK2), a key gene in the glycolytic pathway. 49 Furthermore, Fu et al proposed that highly expressed WTAP promotes aberrant mRNA methylation, inhibiting the transcription and translation of the target gene early growth response 3 (EGR3), and should be considered a potential marker for OC subtype diagnosis. 50
Ovarian cancer and VIRMA
VIRMA, also known as KIAA1429, is a recently identified m6A methyltransferase that is highly amplified or mutated in various cancer types, indicating its potential involvement in cancer development. Studies have shown that VIRMA is transcriptionally activated by SPI1, highly expressed in OC, and enhances the stability of enolase1 (ENO1) mRNA in an m6A-dependent manner, thereby promoting aerobic glycolysis and contributing to OC development. 51
Ovarian Cancer and “Readers”
The m6A-modified RNAs exert diverse biological functions through the recognition of m6A-binding proteins, known as “readers”, which directly bind to m6A-modified sites. 52 YT521-B homology (YTH) domain family (YTHDF1, YTHDF2, YTHDF3, YTHDC1, and YTHDC2), were the first m6A recognition proteins attracting attention. In addition, heterogeneous nuclear ribonucleoprotein (HNRNP) family (HNRNPPA2B1, HNRNPC, and HNRNPG), insulin-like growth factor 2 mRNA-binding proteins 1/2/3 (IGF2BP1/2/3), and eukaryotic initiation factor 3 (eIF3) function as “readers” and mediate tumor development through different mechanisms.7,53 The functions and mechanisms of m6A binding proteins on OC are as follows (Table 2 and Figure 2).
Table 2.
Roles of RNA-binding Proteins in Ovarian Cancer (OC).
| Readers | Genes Regulated | Mechanisms | Roles in OC | Year | Reference |
|---|---|---|---|---|---|
| YTHDF1 | EIF3C | Enhances EIF3C translation in an m6A-dependent manner, impacting total protein translation | Promotes OC proliferation, migration, and invasion OC | 2020 | 55 |
| YTHDF1 | TRIM29 | Enhances TRIM29 translation by binding to TRIM29 mRNA | Promoting CSCs-like features in drug-resistant OC cells | 2021 | 56 |
| YTHDF1 | RIPK4 | Enhances RIPK4 mRNA stability, thus upregulating RIPK4 via METTL3-mediated m6A modification and activating the NF-κB signaling pathway | Promotes proliferation and cisplatin resistance in OC | 2024 | 32 |
| YTHDF2 | BMF | FBW7-induced YTHDF2 degradation accelerates m6A-modified BMF mRNA decay | Promotes proliferation, migration, and invasion and inhibits apoptosis in OC | 2021 | 57 |
| YTHDF2 | miR-145 | A negative feedback loop is formed between YTHDF2 and miR-145 | Promotes proliferation, migration, and invasion and inhibits apoptosis in OC | 2020 | 58 |
| YTHDF2 | MEG3 | METTL3/YTHDF2-mediated MEG3 decay promotes OC proliferation and metastasis via the miR-885-5p/VASH1 axis | Promotes proliferation and metastasis in OC | 2024 | 35 |
| IGF2BP1 | circPLPP4 | METTL3/IGF2BP1 stabilizes and upregulates circPLPP4 so that it sponges the miR-136/PIK3R1 axis to confer CDDP resistance | Promotes cisplatin resistance in OC | 2024 | 33 |
| IGF2BP1 | SRF | Enhances SRF and its target gene expression post-transcriptionally | Promotes OC proliferation, migration, and invasion | 2019 | 60 |
| IGF2BP1 | UBA6-AS1 | Promotes lncRNA UBA6-AS1 expression and stability | Inhibits OC proliferation, migration, and invasion | 2022 | 61 |
| IGF2BP1 | BIRC5 | METTL3 and IGF2BP1 both regulate BIRC5 expression and stability through m6A modification | Promotes cisplatin resistance in OC | 2023 | 31 |
| IGF2BP2 | SNAI1 | Reduced FTO expression increases SNAI1 mRNA m6A modification, enhancing IGF2BP2 binding to SNAI1 mRNA and SNAI1 stability | Promoting proliferation, migration and invasion of OC | 2022 | 62 |
| IGF2BP2 | VGLL1 | METTL3-mediated m6A modification contributes to VGLL1 upregulation via IGF2BP2 recognition-dependent manner | Promotes OC proliferation and metastasis | 2024 | 34 |
| IGF2BP1/2/3 | circNFIX | IGF2BP1/2/3 co-recognition promotes CircNFIX expression, acting as a miRNA sponge and upregulating PD-L1 and activating JAK1/STAT3 | Promoting proliferation, migration and invasion and inhibiting apoptosis in OC | 2023 | 63 |
| HNRNPA2B1 | CDK19 | miR-30c-5p regulates HNRNPA2B1, reducing CDK19 mRNA stability and m6A levels | Promotes OC proliferation, migration, and invasion | 2023 | 65 |
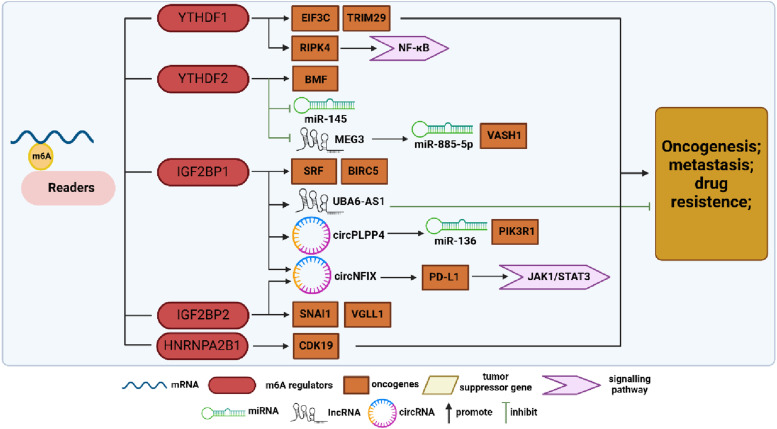
Figure 2.
Summary diagram illustrating m6A-binding protein regulation in ovarian cancer (OC) development. M6A-modified RNAs interact with m6A-binding proteins, exerting various biological functions. This review primarily summarizes the research on YTHDF1, YTHDF2, IGF2BP1, IGF2BP2, and HNRNPA2B1 in OC. YTHDF1 enhances EIF3C and TRIM29 translation and stabilizes RIPK4 expression, while YTHDF2 promotes BMF expression and suppresses miR-145 and lncMEG3, facilitating OC progression. IGF2BP1 and IGF2BP2 promote SRF, BIRC5, SNAI1, VGLL1, circPLPP4, and circNFIX expression, promoting the malignant development of OC. However, that the context-dependent regulation of OC by IGF2BP1 is exemplified by its positive modulation of lncUBA6-AS1, which exerts anticancer effects. HNRNPA2B1 regulates CDK19 expression, further promoting OC progression.
Ovarian cancer and YTH domain family
YTHDF1–3 are highly homologous proteins that recognize and bind to m6A modification sites through the C-terminal YTH domain. However, they diverge in their main functions: YTHDF1 interacts with translation initiation factors, inducing target mRNA translation; YTHDF2 affects target mRNA stability; and YTHDF3 modulates translation and decay of m6A-modified mRNAs by interacting with YTHDF1 and YTHDF2. 54 Liu’s team showed that YTHDF1 promoted ovarian carcinogenesis and metastasis by binding to the m6A-modified protein translation initiation factor EIF3C mRNA, thereby enhancing EIF3C translation in an m6A-dependent manner, ultimately affecting total protein translation. 55 Hao et al demonstrated that YTHDF1 is recruited by m6A-modified tripartite motif protein 29 (TRIM29) and promotes TRIM29 translation in cisplatin-resistant OC cells. Knockdown of YTHDF1 in these cells suppresses their cancer stem cell (CSC)-like features, which can be reversed by TRIM29 overexpression, suggesting a dependency on m6A-YTHDF1 for its oncogenic role. 56 Furthermore, Xu et al revealed the clinical significance of a novel regulatory axis involving the tumor suppressor F-box and WD repeat domain-containing 7 (FBW7), which mediates the ubiquitination and subsequent proteasomal degradation of multiple oncogenic proteins, in OC. This regulatory axis is known as the FBW7-YTHDF2-BMF (Bcl-2 modifying factor) axis. FBW7 antagonizes YTHDF2, leading to the inhibition of its destabilizing effect on the m6A-modified pro-apoptotic BMF mRNA, which ensures the stable expression of BMF mRNA, consequently hindering OC growth and progression. 57 Li et al found that YTHDF2 expression was significantly up-regulated in OC, where it disrupted miR-145 via a bidirectional negative feedback loop, promoting the proliferation and migration of OC cells. 58 These findings underscore the multifaceted role of the YTH domain family in driving the malignant progression of OC through multiple mechanisms. However, significant uncharted territory remains in the exploration of the role of YTH domain family in OC.
Ovarian cancer and IGF2BPs
The IGF2BP family exhibits high structural similarity and plays crucial roles in regulating mRNA stability, translation, and localization. 59 Upregulation of IGF2BP proteins has been associated with poor prognosis in malignant tumors such as breast, colorectal, glioma, hepatocellular, and pancreatic cancers. Specifically, IGF2BP1 recognizes and binds to m6A-modified serum response factor (SRF) mRNA, thereby enhancing its transcriptional activation and maintaining the expression of various IGF2BP1-SRF target genes, which in turn contributes to tumor growth and invasion, while also promoting CSC-like tumor cell characteristics. Müller et al observed the role of this mechanism in ovarian, hepatocellular, and lung cancers, correlating with poor prognosis and low overall survival. 60 Wang et al found that an OC-inhibiting lncRNA called ubiquitin-like modifier-activating enzyme 6 antisense RNA 1 (UBA6-AS1) increased the m6A level of its target UBA6 mRNA by recruiting RBM15. They further noted an increase in the stability of UBA6-AS1 upon IGF2BP1 binding, which in turn suppressed the malignant phenotype of OC. 61 Additionally, Sun et al demonstrated that IGF2BP2 recognized and bound to m6A modification sites, promoting mRNA stability, and that FTO inhibited SNAI1 mRNA expression in an m6A-IGF2BP2-dependent manner, thereby attenuating OC growth and metastasis. 62 Furthermore, M6A-circNFIX, which is highly expressed in OC under the coregulation of IGF2BP1/2/3, up-regulates the expression of PD-L1 and activates the JAK1/STAT3 pathway via circNFIX/miR-647/IL-6R, thereby promoting OC proliferation, metastasis, and immune escape. 63 The aforementioned studies suggest that IGF2BPs contribute to OC progression primarily through their roles in promoting the transcriptional activity of target mRNAs and enhancing their stability.
Ovarian cancer and HNRNPs
The HNRNP family differs from the aforementioned “readers” in that these proteins do not exclusively recognize m6A-modified sites through direct binding. For example, HNRNPC and HNRNPG preferentially bind to m6A-modified RNAs through an “m6A-switch” mechanism. 64 HNRNPA2B1 is highly expressed in OC tissues, garnering attention for its role as an oncogene in OC development. Chen et al identified HNRNPA2B1 as a potential target of miR-30c-5p in OC. They found that miR-30c-5p inhibition led to decreased CDK19 mRNA stability and reduced m6A levels, thereby inhibiting OC progression. 65
Ovarian Cancer and “Erasers”
“Erasers”, as their name suggests, refer to demethylases responsible for the elimination of m6A modifications and mainly include fat mass and obesity-associated protein (FTO) and alkB homolog 5 (ALKBH5). 66 Their presence indicates the dynamic reversible nature of m6A modifications, and also significantly influences the biological characteristics of OC (Table 3 and Figure 3).
Table 3.
Roles of Demethylases in Ovarian Cancer (OC).
| Erasers | Genes Regulated | Mechanisms | Roles in OC | Year | Reference |
|---|---|---|---|---|---|
| FTO | PDE4B, PDE1C | Inhibits the stable expression of PDE4B and PDE1C by eliminating m6A modifications | Inhibits OC proliferation, migration, and invasion | 2020 | 71 |
| FTO | ATG5, ATG7 | circRAB11FIP1 promotes autophagy via FTO-mediated demethylation of ATG5 and ATG7 | Unclear | 2021 | 72 |
| FTO | SNAI1 | Reduced FTO expression increases m6A modification of SNAI1 mRNA, enhancing its stability and recognition and binding by IGF2BP2 | Inhibits OC proliferation, migration, and invasion | 2022 | 62 |
| FTO | NNMT | Promotes DNA breaks and mediates the response to cisplatin by modulating m6A-NNMT | Inhibits drug resistance in OC | 2023 | 73 |
| FTO | RP5-991G20.1 | Regulates drug resistance through the RP5-991G20.1/hsa-miR-1976/MEIS1 signaling pathway | Inhibits proliferation and drug resistance in OC | 2023 | 74 |
| FTO, ALKBH5 | FZD10 | Downregulation of FTO and ALKBH5 increases m6A modifications in FZD10 mRNA, leading to up-regulate Wnt signaling and PARPi resistance | Inhibiting PARPi resistance in OC | 2019 | 77 |
| ALKBH5 | HuR, Bcl-2 | ALKBH5 promotes the EGFR-PI3K-AKT-mTOR and Bcl-2/Beclin1 signaling pathways by enhancing the stability of HuR and Bcl-2 mRNA respectively | Promotes OC proliferation, migration, invasion | 2019 | 76 |
| ALKBH5 | JAK2 | ALKBH5-HOXA10 promotes stable expression of JAK2 thereby activating the JAK2/STAT3 signaling pathway | Promotes drug resistance in OC | 2021 | 78 |
| ALKBH5 | ITGB1 | ALKBH5 promotes ITGB1 mRNA expression in a YTHDF2-m6A-dependent manner, triggering FAK and Src phosphorylation | Promoting lymphangiogenesis and metastasis in OC | 2023 | 79 |
| ALKBH5 | NANOG | TLR4-NF-κB-upregulates ALKBH5, leading to the demethylation and stabilization of NANOG in OC cells co-cultured with M2 macrophages | Promotes proliferation, migration, and invasion, and inhibits apoptosis in OC | 2020 | 80 |
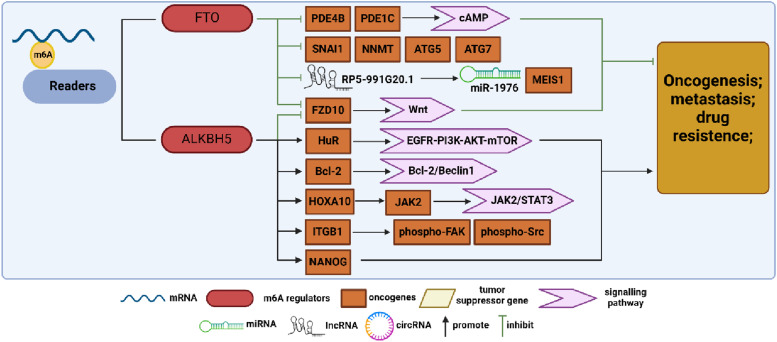
Figure 3.
Summary diagram illustrating m6A demethylase regulation in ovarian cancer (OC) development. FTO and ALKBH5 are key demethylases that modulate biological functions by removing m6A modifications. In OC research, FTO acts as a tumor suppressor gene, inhibiting the expression of genes, such as PDE4B, PDE1C, SNAI1, NMMT, ATG5, ATG7, FZD10, and lncRP5-991G20.1, by eliminating m6A modifications, thus suppressing OC progression. Conversely, ALKBH5 exhibits contrasting effects, promoting OC progression by upregulating the expression of HuR, Bcl-2, JAK2, ITGB1, and NANOG.
Ovarian cancer and FTO
Initially identified for its involvement in adipogenesis and obesity, 67 FTO was later found to have demethylase activity. As the first m6A demethylase discovered, FTO is highly expressed and functions as an oncogene in various malignant tumors such as acute myeloid leukemia, pancreatic cancer, hepatocellular carcinoma, and non-small-cell lung cancer (NSCLC).68-70 However, this was not exactly the case in OC. Huang et al demonstrated that FTO acts as an OC suppressor and inhibits the CSC-like features of OC. They further identified 2 phosphodiesterase genes, PDE4B and PDE1C, as downstream FTO targets that regulate the cAMP signaling pathway and play a key role in maintaining ovarian CSC-like features. 71 Based on previous studies showing the involvement of m6A in autophagy, Zhang et al found that CircRAB11FIP1 binds to and upregulates FTO mRNA expression, thereby modulating the m6A methylation levels of ATG5 and ATG7 mRNA through FTO. This mechanism ultimately enhances autophagy and malignant behavior in OC. 72 Additionally, Sun et al demonstrated that decreased FTO expression elevates m6A levels in SNAI1 mRNA, upregulating SNAI1 expression and consequently promoting OC proliferation, invasion, and migration. 62 Furthermore, Huang et al revealed that FTO overexpression enhances OC sensitivity to platinum and induces DNA double-strand breaks, with nicotinamide N-methyltransferase (NNMT) emerging as a novel FTO target regulating OC response to platinum in an m6A-dependent manner. 73 Tumor-associated long non-coding RNAs (lncRNAs) modulated by epigenetic modification switches play pivotal roles in immune evasion and chemoresistance in OC. Chen et al developed a prognostic model integration methylation-associated and immune-associated lncRNAs, revealing that FTO inhibits OC proliferation and drug resistance by regulating the RP5-991G20.1/hsa-miR-1976/MEIS1 signaling pathway. 74 Several studies have confirmed FTO’s role as a suppressor influencing various malignant behaviors in OC, including proliferation, metastasis, autophagy, and drug resistance, through diverse ontogenetic mechanisms.
Ovarian cancer and ALKBH5
ALKBH5, another significant m6A demethylase, modulates various biological processes, such as nuclear RNA export, metabolism, and gene expression, with its involvement in different RNA interactions playing distinct roles across various cancer types. 75 In ovarian cancer, Zhu et al identified ALKBH5 overexpression in tumor tissues, correlating with cancer progression through the inhibition of autophagy in OC cells. 76 In contrast, ALKBH5, in conjunction with FTO, contributes to PARP inhibitor resistance in BRCA1/2 mutant OC cells by regulating frizzled class receptor 10 (FZD10) mRNA expression and mediating the Wnt signaling pathway. 77 Liu et al observed upregulated ALKBH5 expression in cisplatin-resistant OC, leading to enhanced OC cell proliferation and cisplatin resistance both in vivo and in vitro. They identified homeobox A10 (HOXA10) as a transcription factor promoting ALKBH5 transcription, reciprocally regulated by ALKBH5. Further analysis revealed that the sustained upregulation of ALKBH5-HOXA10 promoted OC tumor growth and cisplatin resistance via m6A-dependent activation of the JAK2/STAT3 signaling pathway. 78 Sun et al found that hypoxia could stimulate the expression of hypoxia inducible factor 1 subunit alpha (HIF-1α) and ALKBH5. They observed that ALKBH5 regulated the expression of integrin subunit beta 1 (ITGB1) in an m6A-YTHDF2-dependent manner, which triggered the phosphorylation of focal adhesion kinase (FAK), thus promoting OC lymphangiogenesis and metastasis. 79 In the field of immunology, Jiang et al established an in vitro model by co-culturing M1 and M2 macrophages with OC cells and found that this co-culture significantly increased tumor cell invasiveness. Additionally, they observed that Toll-like receptor 4 (TLR4) upregulated the expression of ALKBH5 in M2 co-cultured group through activation of the NF-κB signaling pathway. ALKBH5, in turn, promoted OC development by demethylating Nanog homeobox (NANOG). 80
M6A Regulators and Ovarian Cancer Prognosis
Since m6A modification is involved in OC tumorigenesis and progression through multiple mechanisms, m6A methylation regulators play a crucial role in tumor prognosis. Fan et al identified IGF2BP1, VIRMA, and ZC3H13 as the most prognostically predictive m6A-associated regulatory proteins, as evidenced by risk model construction. Gene Set Enrichment Analysis (GSEA) further supported the prognostic significance of these proteins by associating them with cancer pathways and the Wnt signaling pathway, thus corroborating their prognostic value. 81 Li et al analyzed the prognostic value of each m6A-modified regulatory factor, identifying VIRMA, IGF2BP1, and HNRNPA2B1 through the Lasso Cox regression analysis and constructed a risk scoring model based on the above 3 factors. Additionally, they constructed a regulatory network of miRNAs, m6A regulators, and m6A target genes and conducted preliminary experimental validation of the miR-196b-5p-IGF2BP1-PTEN pathway within this network, with the results providing some degree of reliability for their constructed regulatory network. 82 Zhang et al analyzed the correlation between each m6A regulator and clinical characteristics of OC (including age, grade, stage, and tumor status). Using univariate and multivariate Cox regression analyses, they identified HNRNPA2B1, KIAA1429, and WTAP as significantly linked to overall survival, leading to the construction of a risk scoring model. Additionally, they observed a correlation between immune infiltration and these 3 m6A regulators. 83 Using the TCGA database, Han et al conducted a bioinformatics analysis, revealing that nearly all OC patients (99.31%) exhibited mutations or copy number variants (CNVs) in at least one m6A-regulated gene, with high WTAP expression correlating significantly with poorer prognosis. 84 Using GEO dataset, Wang et al analyzed the correlation between the altered expression levels of multiple m6A regulators and the prognostic regression of OC, emphasizing the potential involvement of m6A regulators (RBM15 B, ZC3H13, YTHDF1, and IGF2BP1) in immune cell infiltration and immune evasion in OC. 85 Conversely, Tan et al analyzed various OC datasets from TCGA and identified 7 m6A regulators—CBLL1, FTO, HNRNPC, METTL3, METTL14, WTAP, ZC3H13, RBM15B, and YTHDC2—associated with poorer overall survival in OC. They also identified optimal populations that might benefit from immunotherapy. 86 Furthermore, using the TCGA database, Zhu et al found that high expression of KIAA1429 and YTHDC2 correlated with poorer prognosis in OC. They constructed a prognostic model based on 5 m6A RNA methylation regulators in OC (KIAA1429, YTHDC2, ZC3H13, WTAP, and FTO) that are highly expressed in OC. 87
Overall, bioinformatics analyses have been widely applied m6A-related studies concerning OC prognosis (Table 4). However, discrepancies exist among the results obtained using different analytical tools. Moreover, limited experimental validations have been performed, leading to broad and superficial conclusions. Further in-depth studies are necessary to elucidate the specific mechanisms of individual regulators.
Table 4.
Roles of m6A Regulators in Ovarian Cancer (OC) Prognosis.
| m6A Regulators | Data Sources | Analytical Method | Year | Reference |
|---|---|---|---|---|
| IGF2BP1, VIRMA, ZC3H13 | TCGA (RNA-seq data for 379 patients with OC and clinicopathologic information for 587 patients with OC), GTEx (RNA-seq data of 88 normal human ovarian tissues) | Univariate and multivariate Cox regression analyses | 2020 | 81 |
| VIRMA, IGF2BP1, HNRNPA2B1 | GDC (RNA-seq data and corresponding clinical data for 374 patients with OC), GTEx (RNA-seq data from 88 cases of normal human ovarian tissues) | Lasso Cox regression analysis | 2021 | 82 |
| HNRNPA2B1, KIAA1429, WTAP | TCGA (RNA-seq and clinicopathological data of 379 patients with OC GTEx (RNA-seq data from 88 cases of normal human ovarian tissues) | Univariate and Lasso Cox regression analyses | 2021 | 83 |
| WTAP | TCGA (mutation and CNV data for 579 patients with OC) | Multivariate Cox regression analysis | 2020 | 84 |
| RBM15B, ZC3H13, YTHDF1, IGF2BP1 | TCGA, GEO (GSE14407, GSE12470, GSE69428, GSE84829, GSE28979, GSE9891, GSE73168, GSE30587, GSE51373) | One-way ANOVA, Spearman’s correlation test | 2021 | 85 |
| CBLL1, FTO, HNRNPC, METTL3, METTL14, WTAP, ZC3H13, RBM15B, YTHDC2 | TCGA, GEO (GSE140082, GSE13876), GTEx | Univariate and Lasso Cox regression analyses | 2022 | 86 |
| KIAA1429, YTHDC2, ZC3H13, WTAP, FTO | TCGA (RNA-Seq profiles and clinical data of 379 patients with OC), UCSC (RNA-Seq profiles of 379 OC tissues and 88 healthy ovarian tissues), GEO (GSE66957, GSE63885) | Univariate Cox regression analysis | 2022 | 87 |
Ovarian Cancer and Potential m6A-associated Targeted Drugs
As m6A research continues to advance, there is a burgeoning interest in exploring the therapeutic potential of pharmacological agents targeting m6A-associated regulatory factors for cancer treatment. However, current research into m6A-targeted drugs remains in its infancy, primarily focusing on cancers other than OC.
As the core component of the m6A methyltransferase complex, METTL3 plays a crucial role in tumorigenesis and development, making it a promising therapeutic target for anticancer drugs. Yankova et al reported the first highly potent and selective inhibitor of METTL3, named STM2457, demonstrating its anti-leukemic efficacy in vivo. 88 Furthermore, a newly developed oral small-molecule METTL3 inhibitor named STC-15 has received IND approval for its Phase 1 clinical study in cancer patients, which began in November 2022. 89 In a recent study, orthotopic NAFLD-HCC tumors were treated with STM2457 in vivo, and it was found that STM2457 significantly inhibited tumor growth and synergized with anti-PD-1 therapy, leading to a strong induction of IFN-γ+ and GZMB+ CD8+ T cell infiltration. 90 Adenosine, a SAM-competitive inhibitor of METTL3, has been identified as one of the 2 moieties of SAM. 91 Moroz-Omori et al characterized a nanomolar inhibitor of METTL3 (UZH1a) by screening an adenine-based library and performing a homogenous time-resolved fluorescence (HTRF) enzyme inhibition assay. Further in vitro validation showed a dose‐dependent reduction in m6A methylation levels. 92 In NSCLC, chidamide decreases the stability and translational efficiency of WTAP and METTL3 mRNAs, thereby downregulating c-MET expression and enhancing NSCLC cell sensitivity to crizotinib. 93
Inhibitors targeting m6A “readers” have limited coverage. For example, salvianolic acid C (SAC), a small-molecule inhibitor of YTHDF1, has been reported to reverse fragile X syndrome, 94 but no YTHDF1 inhibitors for cancer therapy have been described yet. Additionally, Wang et al reported that higher levels of IGF2BP2 in patients with AML, particularly in leukemic stem cells/leukemic initiating cells (LSCs/LICs), are associated with poorer prognosis. Subsequently, they identified a compound, CWI1-2, which directly interacts with IGF2BP2 and shows promising anti-leukemic efficacy both in laboratory and animal models. Remarkably, CWI1-2 achieves this therapeutic effect by inhibiting IGF2BP2’s m6A reader activity, with minimal adverse effects. 95 In the field of OC research, the availability of targeted drugs against m6A regulators is limited. Chang Su reported that AE-848, a small molecule targeting IGF2BP3, could be an effective treatment for OC. Through a subcutaneous tumor model, AE-848 has been validated to inhibit tumor growth by reducing the expression of tumor-associated antigens (c-MYC/VEGF/Ki67/CDK2) and enhancing the anti-tumor effect of macrophages. 96
Among the many m6A regulators, FTO stands out as the most promising target for inhibitor development. Over 10 FTO inhibitors have been discovered to date, with their effectiveness validated in various types of tumors. One early-identified FTO inhibitor, meclofenamic acid (MA), exhibits high selectivity by competing for FTO binding sites. 97 Its ethyl ester derivative, MA2, has been shown to suppress glioblastoma stem cell (GSC) growth and self-renewal in vitro, along with inhibiting tumor growth in vivo. Moreover, the combination of MA2 with the chemotherapy drug temozolomide (TMZ) has shown a synergistic effect in suppressing glioma cells. 98 In 2022, Liu et al encapsulated MA into γ-cyclodextrin cavity and developed a photothermal immunotherapy based on gold nanoparticles. This MA-nanodrug specifically targeted prostate tumor cells and selectively accumulated at the tumor site in vivo by increasing CD8+ T cell infiltration while inactivating PD-L1. 99 FB23-2, an improved selective FTO inhibitor synthesized based on MA, exhibits superior anti-proliferation activity and promotes myeloid differentiation and apoptosis in acute myeloid leukemia (AML) by regulating the expression of RARA and ASB2. The patient-derived xeno-transplantation (PDX) AML mouse model further shows its safety and therapeutic efficacy in vivo. 100 Additionally, in a recent study on hepatocellular carcinoma (HCC), FB23-2 was loaded into a nanocarrier for targeted release into tumor-infiltrating dendritic cells (TIDCs) along with tumor-associated antigens (TAA) to investigate its efficacy in HCC. Findings from the in vivo study showed that intratumoral injection of the nanodrug promoted the maturation of dendritic cells (DCs), improved tumor infiltration of effector T cells, and generated immune memory, synergistically inhibiting distant tumor growth and lung metastasis with immune-checkpoint blockade (ICB) therapy. 101 Su et al identified CS1 and CS2 as efficacious FTO inhibitors, exhibiting potent anti-leukemic efficacy by enhancing AML cell sensitivity to T cell cytotoxicity and overcoming immune evasion. 102 Epigallocatechin gallate, a tea flavonoid, exhibits powerful antioxidant, anti-inflammatory, and anticancer effects, likely attributed to the m6A-dependent regulation of cyclin A2 and CDK2 through the downregulation of FTO and upregulation of YTHDF2. 103 FTO-04, which was developed based on the mechanistic structure of FTO, has been found to significantly inhibit patient-derived GSC neurosphere formation without affecting healthy human neural stem cell growth. 104 Dac51, a more potent FTO inhibitor developed by the same group that developed FB23-2, shares a similar mechanism with CS1 and CS2. Dac51 treatment increases CD8+ T cell infiltration and synergizes with anti-PD-L1 blockade. 105 Additionally, Malacrida et al revealed that imidazobenzoxazin-5-thione MV1035, initially synthesized as a sodium channel blocker, exhibits ALKBH5 inhibition and increases m6A levels through off-target interactions and further suppresses U87 glioblastoma cell migration and invasiveness. 106 Another specific inhibitor of ALKBH5, named ALK-04, was found to enhance anti-PD-1 treatment through promotion of immune cell accumulation in the tumor microenvironment. 107
In summary, m6A-targeted drugs show significant feasibility and promise in cancer therapy. Moreover, the aforementioned drug studies indicate that targeting m6A regulatory factors may mediate immune infiltration and potentially synergize with immune checkpoint inhibitor (ICI) immunotherapy. However, current research data are limited, with most targeted drug studies primarily focusing on glioma, AML, and HCC, and notably scarce research on other tumors, including OC. This underscores the need for further exploration in this area as it presents a promising avenue for further exploration.
Discussion and Perspectives
OC ranks third among gynecological tumors but has the highest mortality rate. Owing to the absence of typical early clinical symptoms, most patients (70%) are diagnosed at advanced stages. Currently, the treatment to OC primarily relies on surgery, complemented by radiotherapy, chemotherapy, targeted therapy, and other therapeutic means, yet long-term survival rates remain largely unchanged. 108 Therefore, the urgent focus of current OC research is to identify more accurate early diagnostic markers and therapeutic targets. In recent years, that the significance of m6A modification, one of the most prevalent RNA modifications in eukaryotes, has emerged in post-transcriptional regulation of gene expression. Notably, m6A-related regulators are implicated in the progression and prognosis of various tumors. Current studies have highlighted m6A as a “double-edged sword”, regulating oncogenes or suppressor genes through various methyltransferases, m6A binding proteins, and demethylases, which are involved in the regulation of growth, proliferation, migration, invasion, metastasis, drug resistance, and prognosis of OC.
The majority of m6A studies have focused on its regulatory factors in various aspects of OC, including proliferation, metastasis, and drug resistance. Our review consolidates the specific mechanisms of action of diverse m6A-associated regulatory factors in these areas (Figure 4). Among them, METTL3 and WTAP facilitate m6A modification by promoting the expression of oncogenic mRNAs or non-coding RNAs (ncRNAs), while concurrently suppressing the expression of tumor suppressor genes, ultimately promoting the proliferation and metastasis of OC. However, METTL14 has been observed to impede OC proliferation by reducing the stability of TROAP mRNA. Yet, research on METTL14 remains limited, and the generalizability of its findings warrants further verification. Another less-explored methyltransferase, VIRMA, activated by SPI1, enhances ENO1 mRNA stability in an m6A-dependent manner, thereby contributing to proliferation, migration, and invasion effects in OC. In the “readers” section, we provide an overview of the specific mechanisms of various m6A-binding proteins, such as YTHDF1, YTHDF2, IGF2BP1, IGF2BP2, and HNRNPA2B1, in tumor proliferation and metastasis. We elucidated these mechanisms from perspectives such as mRNA, ncRNAs, and downstream signaling pathways. In “erasers”, the primary subjects of research concerning metastasis and proliferation are FTO and ALKBH5, with both exhibiting contrasting roles in OC development. FTO inhibits OC migration and proliferation by removing m6A modifications, thereby stabilizing the expression of PDE4B and PDE1C. Conversely, ALKBH5 enhances OC proliferation, migration, and invasion through multiple signaling pathways. Although research on drug resistance is limited, the overall roles of these regulatory factors appear to parallel their functions in proliferation and metastasis. METTL3-mediated m6A modification upregulates RIPK4, with YTHDF1 enhancing RIPK4 mRNA stability, subsequently activating the NF-κB signaling pathway to confer OC resistance to cisplatin. Additionally, METTL3 and IGF2BP1 regulate BIRC5 and circPLPP4 expression and stability through m6A modification, thereby promoting OC resistance to cisplatin. Conversely, downregulation of FTO and ALKBH5 increases m6A modifications in FZD10 mRNA, leading to upregulation of the Wnt signaling pathway and reduced resistance to PARPi, ultimately inhibiting PARPi resistance in OC. The synergistic effects between these complexes play a pivotal role in drug resistance. Furthermore, there is a significant scarcity of cross-research between m6A-associated regulatory factors and other phenotypes of OC, such as immune evasion, immune infiltration, autophagy, metastasis, and others. Considering the potential significance of these areas for clinical treatment, we advocate for continued exploration of these areas in future studies.
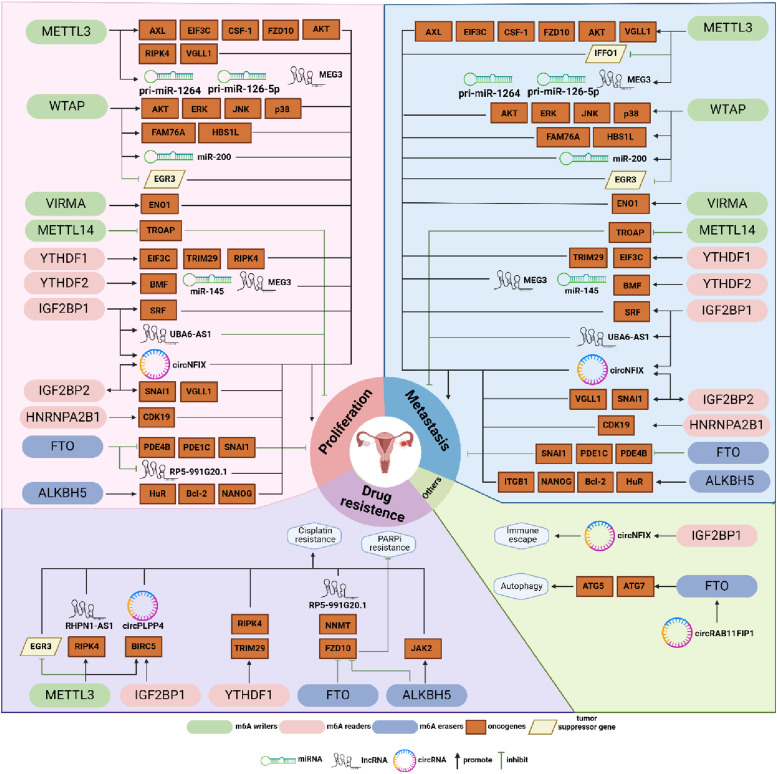
Figure 4.
Summary diagram illustrating the effects of m6A-related regulatory factors on various clinical phenotypes of ovarian cancer (OC). This review categorizes and summarizes experimental studies, identifying 4 main clinical phenotypes of OC regulated by m6A modifications: proliferation, metastasis, drug resistance, and others. Most studies indicate the roles of m6A-related regulatory factors in regulating OC proliferation and metastasis. Apart from FTO and METTL14, most factors promote these processes. Several studies have elucidated the regulatory mechanisms of METTL3, IGF2BP1, YTHDF1, FTO, and ALKBH5 in OC drug resistance, with conclusions largely parallel to those regarding proliferation and metastasis. However, research on immune-related phenotypes, metabolism, autophagy, and other aspects remains limited. Only IGF2BP1 has been reported to promote immune evasion by upregulating circNFIX, while circRAB11FIP1 promotes autophagy through FTO-mediated upregulation of ATG5 and ATG7.
Overall, methyltransferases, including METTL3, WTAP, VIRMA, and m6A-binding proteins YTHDFs consistently play pro-carcinogenic roles in OC, whereas the demethylase FTO is downregulated and plays a cancer-suppressing role. However, the roles of other regulators in OC show pleiotropy and contradiction, potentially attributed to the differences in upstream and downstream signaling pathways involved in, as well as the complex tumor microenvironment. This paper suggests that further exploration is needed to elucidate the mechanisms underlying this paradoxical phenomenon. Furthermore, the complexity and diversity of m6A modification regulators and related mechanisms have rendered many m6A regulators in OC largely unexplored territory. The heterogeneity and complexity of m6A modification in OC, including the potential synergistic or antagonistic effects of different regulators on the carcinogenesis, warrant further investigation. Beyond mechanistic studies, greater emphasis on the clinical translation of m6A by researchers is also essential.
Early diagnosis has always been a challenge in OC management, and there is a pressing need to explore the potential of m6A-related regulators as biomarkers for early OC detection. While several risk scoring models indicate the potential of m6A-related regulators for prognostic assessment, they are primarily based on analyses of public databases, lacking validation through large-scale, multi-center experiments and clinical trials. Addressing these shortcomings will be crucial in future studies. Meanwhile, certain studies have indicated correlations between m6A-related regulators and OC immune cell infiltration, immune evasion, and immune response, which provides feasibility and theoretical support for immunotherapy in OC. We believe that future studies will achieve significant breakthroughs in this field.
Another significant challenge lies in devising effective treatment strategies for OC, where m6A-targeted therapy holds promise. Currently, METTL3 inhibitors stand out as the sole m6A-targeted drugs in clinical trial stages, indicating their potential as the most promising therapeutic targets, with their research progress and efficacy worth anticipating. While research on FTO inhibitors is relatively extensive, with significant anti-cancer effects demonstrated in both in vitro and in vivo experiments uncertainties prevail. As described in the preceding section on FTO in this paper, its anti-cancer effect in OC is ultimately achieved by reducing the m6A RNA levels. Therefore, whether FTO inhibitors might have a contrary effect in OC and whether transitioning to FTO activators is necessary for effective treatment in OC remain unknown and warrant confirmation in future research. Overall, a wide array of m6A regulatory factors play extensive roles in the regulation of gene expression and signaling pathways, influencing various pathological and physiological processes in the human body. However, given that current research findings are predominantly derived from animal experiments, the safety and potential side effects of m6A-targeted therapy in humans remain uncertain and warrant further evaluation. Consequently, future clinical trials must prioritize rigorous testing and measures to mitigate the risk of in vivo side effects associated with m6A-targeted drugs.
Conclusion
Our review delves into the advancements of m6A RNA modification in OC, elucidating the impact of methyltransferases, m6A-binding proteins, and demethylases on tumor proliferation, metastasis, and drug resistance. These insights underscore the potential significance of m6A modification in the diagnosis, prognostic assessment, and treatment of OC. Investigating m6A regulatory factors as potential molecular targets present a novel approach to addressing this complex malignancy. The development of effective targeting RNA regulatory factors may offer novel therapeutic avenues for managing OC and overcoming recurrent drug resistance in the future.
Appendix.
Abbreviations
- ALKBH5
AlkB homolog 5
- AXL
AXL receptor tyrosine kinase
- BMF
Bcl-2-modifying factor
- CCNG2
Cyclin G2
- CSC
Cancer stem cell
- EGR3
Early growth response 3
- eIF3
Eukaryotic initiation factor 3
- EMT
Epithelial-to-mesenchymal transition
- ENO1
Enolase1
- EOC
Epithelial ovarian cancer
- FAK
Focal adhesion kinase
- FAM76A
Family with sequence similarity 76 member A
- FBW7
F-box and WD repeat domain-containing 7
- FTO
Fat mass and obesity-associated protein
- FZD10
Frizzled class receptor 10
- GSEA
Gene set enrichment analysis
- NV
Copy number variants
- HBS1L
HBS1-like translational GTPase
- HIF-1α
Hypoxia-inducible factor 1-alpha
- HK2
Hexokinase 2
- HNRNP
Heterogeneous nuclear ribonucleoprotein
- HOXA10
Homeobox A10
- IFFO1
Intermediate filament family orphan 1
- IGF2BP1/2/3
Insulin-like growth factor-2 mRNA-binding proteins 1/2/3
- ITGB1
Integrin subunit beta-1
- lncRNA
Long non-coding RNA
- m6A
N6-methyladenosine
- METTL14
Methyltransferase-like 14
- METTL3
Methyltransferase-like 3
- NNMT
Nicotinamide N-methyltransferase
- OC
Ovarian cancer
- OS
Overall survival
- PTEN
Phosphatase and tensin homolog
- RBM15/15B
RNA-binding motif protein 15/15B
- SAM
S-adenosylmethionine
- SRF
Serum response factor
- TLR4
Toll-like receptor 4
- TRIM29
Tripartite motif protein 29
- TROAP
Trophinin-associated protein
- UBA6-AS1
Ubiquitin-like modifier-activating enzyme 6 antisense RNA 1
- VIRMA
Vir-like m6A methyltransferase associated
- WTAP
Wilms’ tumor 1-associated protein
- YTH
YT521-B homology
- ZC3H13
Zinc finger CCCH-type containing 13
Footnotes
Author Contributions: All authors contributed to study conception and design. YHZ, YFL, YZ and XS were responsible for material preparation, reference collection and interpretation. YHZ and YFL wrote the first draft of the manuscript. FRS, JHZ and YGC read and revised the format. YZG, FY and JW reviewed and revised the manuscript. JW supervised the project. All authors read and approved the final manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The present study was supported by the grants from the Project of Suzhou Science and Technology Development Plan (No. SLJ202006), the Project of Jiangsu Health Development Research Center (No. JSHD2022067), as well as the Fundamental Research Funds for Soochow University (No. H230337).
ORCID iD
Yuhong Zhang https://orcid.org/0009-0003-9059-1640
References
- 1.Stewart C, Ralyea C, Lockwood S. Ovarian cancer: an integrated review. Semin Oncol Nurs. 2019;35(2):151-156. doi: 10.1016/j.soncn.2019.02.001 [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17-48. doi: 10.3322/caac.21772 [DOI] [PubMed] [Google Scholar]
- 3.Desrosiers R, Friderici K, Rottman F. Identification of methylated nucleosides in messenger RNA from Novikoff hepatoma cells. Proc Natl Acad Sci U S A. 1974;71(10):3971-3975. doi: 10.1073/pnas.71.10.3971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Narayan P, Rottman FM. An in vitro system for accurate methylation of internal adenosine residues in messenger RNA. Science. 1988;242(4882):1159-1162. doi: 10.1126/science.3187541 [DOI] [PubMed] [Google Scholar]
- 5.Roundtree IA, Evans ME, Pan T, He C. Dynamic RNA modifications in gene expression regulation. Cell. 2017;169(7):1187-1200. doi: 10.1016/j.cell.2017.05.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun T, Wu R, Ming L. The role of m6A RNA methylation in cancer. Biomed Pharmacother. 2019;112:108613. doi: 10.1016/j.biopha.2019.108613 [DOI] [PubMed] [Google Scholar]
- 7.Zhang L, Hou C, Chen C, et al. The role of N(6)-methyladenosine (m(6)A) modification in the regulation of circRNAs. Mol Cancer. 2020;19(1):105. doi: 10.1186/s12943-020-01224-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Canaani D, Kahana C, Lavi S, Groner Y. Identification and mapping of N6-methyladenosine containing sequences in simian virus 40 RNA. Nucleic Acids Res. 1979;6(8):2879-2899. doi: 10.1093/nar/6.8.2879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niu Y, Zhao X, Wu YS, Li MM, Wang XJ, Yang YG. N6-methyl-adenosine (m6A) in RNA: an old modification with a novel epigenetic function. Genomics Proteomics Bioinformatics. 2013;11(1):8-17. doi: 10.1016/j.gpb.2012.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rottman FM, Bokar JA, Narayan P, Shambaugh ME, Ludwiczak R. N6-adenosine methylation in mRNA: substrate specificity and enzyme complexity. Biochimie. 1994;76(12):1109-1114. doi: 10.1016/0300-9084(94)90038-8 [DOI] [PubMed] [Google Scholar]
- 11.Yang Y, Yan Y, Yin J, et al. O-GlcNAcylation of YTHDF2 promotes HBV-related hepatocellular carcinoma progression in an N(6)-methyladenosine-dependent manner. Signal Transduct Target Ther. 2023;8(1):63. doi: 10.1038/s41392-023-01316-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao W, Qi X, Liu L, Ma S, Liu J, Wu J. Epigenetic regulation of m(6)A modifications in human cancer. Mol Ther Nucleic Acids. 2020;19:405-412. doi: 10.1016/j.omtn.2019.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng S, Han H, Lin S. N(6)-methyladenosine (m(6)A) RNA modification in tumor immunity. Cancer Biol Med. 2022;19(4):385-397. doi: 10.20892/j.issn.2095-3941.2021.0534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y, You Y, Lu Z, et al. N 6 -methyladenosine RNA modification–mediated cellular metabolism rewiring inhibits viral replication. Science. 2019;365(6458):1171-1176. doi: 10.1126/science.aax4468 [DOI] [PubMed] [Google Scholar]
- 15.Wang JN, Wang F, Ke J, et al. Inhibition of METTL3 attenuates renal injury and inflammation by alleviating TAB3 m6A modifications via IGF2BP2-dependent mechanisms. Sci Transl Med. 2022;14(640):eabk2709. doi: 10.1126/scitranslmed.abk2709 [DOI] [PubMed] [Google Scholar]
- 16.Zhou J, Zhu Y, Ai D, et al. Advanced glycation end products impair bone marrow mesenchymal stem cells osteogenesis in periodontitis with diabetes via FTO-mediated N(6)-methyladenosine modification of sclerostin. J Transl Med. 2023;21(1):781. doi: 10.1186/s12967-023-04630-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yao H, Gao CC, Zhang D, et al. scm(6)A-seq reveals single-cell landscapes of the dynamic m(6)A during oocyte maturation and early embryonic development. Nat Commun. 2023;14(1):315. doi: 10.1038/s41467-023-35958-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeng L, Huang X, Zhang J, Lin D, Zheng J. Roles and implications of mRNA N(6) -methyladenosine in cancer. Cancer Commun (Lond). 2023;43(7):729-748. doi: 10.1002/cac2.12458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schöller E, Weichmann F, Treiber T, et al. Interactions, localization, and phosphorylation of the m(6)A generating METTL3-METTL14-WTAP complex. RNA. 2018;24(4):499-512. doi: 10.1261/rna.064063.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qi YN, Liu Z, Hong LL, Li P, Ling ZQ. Methyltransferase-like proteins in cancer biology and potential therapeutic targeting. J Hematol Oncol. 2023;16(1):89. doi: 10.1186/s13045-023-01477-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu J, Yue Y, Han D, et al. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol. 2014;10(2):93-95. doi: 10.1038/nchembio.1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu C, Ren Q, Deng J, Wang S, Ren L. c-MYC/METTL3/LINC01006 positive feedback loop promotes migration, invasion and proliferation of non-small cell lung cancer. Biomed J 2023;100664. doi: 10.1016/j.bj.2023.100664 [DOI] [PubMed] [Google Scholar]
- 23.Wang S, Gao S, Ye W, Li Y, Luan J, Lv X. The emerging importance role of m6A modification in liver disease. Biomed Pharmacother. 2023;162:114669. doi: 10.1016/j.biopha.2023.114669 [DOI] [PubMed] [Google Scholar]
- 24.Hua W, Zhao Y, Jin X, et al. METTL3 promotes ovarian carcinoma growth and invasion through the regulation of AXL translation and epithelial to mesenchymal transition. Gynecol Oncol. 2018;151(2):356-365. doi: 10.1016/j.ygyno.2018.09.015 [DOI] [PubMed] [Google Scholar]
- 25.Ma Z, Li Q, Liu P, Dong W, Zuo Y. METTL3 regulates m6A in endometrioid epithelial ovarian cancer independently of METTl14 and WTAP. Cell Biol Int. 2020;44(12):2524-2531. doi: 10.1002/cbin.11459 [DOI] [PubMed] [Google Scholar]
- 26.Bi X, Lv X, Liu D, et al. METTL3 promotes the initiation and metastasis of ovarian cancer by inhibiting CCNG2 expression via promoting the maturation of pri-microRNA-1246. Cell Death Discov. 2021;7(1):237. doi: 10.1038/s41420-021-00600-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cui S. METTL3-mediated m6A modification of lnc RNA RHPN1-AS1 enhances cisplatin resistance in ovarian cancer by activating PI3K/AKT pathway. J Clin Lab Anal. 2022;36(12):e24761. doi: 10.1002/jcla.24761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang S, Guan H, Lin X, Li N, Geng F, Li J. METTL3 serves an oncogenic role in human ovarian cancer cells partially via the AKT signaling pathway. Oncol Lett. 2020;19(4):3197-3204. doi: 10.3892/ol.2020.11425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y, Qiu JG, Jia XY, et al. METTL3-mediated N6-methyladenosine modification and HDAC5/YY1 promote IFFO1 downregulation in tumor development and chemo-resistance. Cancer Lett. 2023;553:215971. doi: 10.1016/j.canlet.2022.215971 [DOI] [PubMed] [Google Scholar]
- 30.Bi X, Lv X, Liu D, et al. METTL3-mediated maturation of miR-126-5p promotes ovarian cancer progression via PTEN-mediated PI3K/Akt/mTOR pathway. Cancer Gene Ther. 2021;28(3-4):335-349. doi: 10.1038/s41417-020-00222-3 [DOI] [PubMed] [Google Scholar]
- 31.Fan Y, Pan Y, Jia L, et al. BIRC5 facilitates cisplatin-chemoresistance in a m6 A-dependent manner in ovarian cancer. Cancer Med. 2023;13(1):e6811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yin X, Zhao S, Zhang M, et al. m6A-modified RIPK4 facilitates proliferation and cisplatin resistance in epithelial ovarian cancer. Gynecol Oncol. 2024;180:99-110. doi: 10.1016/j.ygyno.2023.11.034 [DOI] [PubMed] [Google Scholar]
- 33.Li H, Lin R, Zhang Y, et al. N6-methyladenosine-modified circPLPP4 sustains cisplatin resistance in ovarian cancer cells via PIK3R1 upregulation. Mol Cancer. 2024;23(1):5. doi: 10.1186/s12943-023-01917-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li H, Cai L, Pan Q, et al. N(6)-methyladenosine-modified VGLL1 promotes ovarian cancer metastasis through high-mobility group AT-hook 1/Wnt/β-catenin signaling. iScience. 2024;27(3):109245. doi: 10.1016/j.isci.2024.109245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Y, Lou S, Zhang J, Zhao S, Lou G. m(6)A methylation-mediated regulation of LncRNA MEG3 suppresses ovarian cancer progression through miR-885-5p and the VASH1 pathway. J Transl Med. 2024;22(1):113. doi: 10.1186/s12967-024-04929-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weng H, Huang H, Wu H, et al. METTL14 inhibits hematopoietic stem/progenitor differentiation and promotes leukemogenesis via mRNA m(6)A modification. Cell Stem Cell. 2018;22(2):191-205.e9. doi: 10.1016/j.stem.2017.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lang F, Singh RK, Pei Y, Zhang S, Sun K, Robertson ES. EBV epitranscriptome reprogramming by METTL14 is critical for viral-associated tumorigenesis. PLoS Pathog. 2019;15(6):e1007796. doi: 10.1371/journal.ppat.1007796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma JZ, Yang F, Zhou CC, et al. METTL14 suppresses the metastatic potential of hepatocellular carcinoma by modulating N(6) -methyladenosine-dependent primary microRNA processing. Hepatology. 2017;65(2):529-543. doi: 10.1002/hep.28885 [DOI] [PubMed] [Google Scholar]
- 39.Panneerdoss S, Eedunuri VK, Yadav P, et al. Cross-talk among writers, readers, and erasers of m(6)A regulates cancer growth and progression. Sci Adv. 2018;4(10):eaar8263. doi: 10.1126/sciadv.aar8263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gu C, Wang Z, Zhou N, et al. Mettl14 inhibits bladder TIC self-renewal and bladder tumorigenesis through N(6)-methyladenosine of Notch1. Mol Cancer. 2019;18(1):168. doi: 10.1186/s12943-019-1084-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu J, Lin H, Wang C, Su Q, Cao B. METTL14-mediated RNA methylation in digestive system tumors. Int J Mol Med. 2023;52(3):86. doi: 10.3892/ijmm.2023.5289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li K, Zhang R, Wei M, et al. TROAP promotes breast cancer proliferation and metastasis. BioMed Res Int. 2019;2019:6140951. doi: 10.1155/2019/6140951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Y, Peng H, Jiang P, et al. Downregulation of methyltransferase-like 14 promotes ovarian cancer cell proliferation through stabilizing TROAP mRNA. Front Oncol. 2022;12:824258. doi: 10.3389/fonc.2022.824258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wei YS, Yao DS, Li L, Lu Y, Yang XM, Zhang WG. [Expression of METTL14 in epithelial ovarian cancer and the effect on cell proliferation, invasion and migration of A2780 and SKOV3 cells]. Zhonghua Fu Chan Ke Za Zhi. 2022;57(1):46-56. doi: 10.3760/cma.j.cn112141-20210925-00553 [DOI] [PubMed] [Google Scholar]
- 45.Ping XL, Sun BF, Wang L, et al. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res. 2014;24(2):177-189. doi: 10.1038/cr.2014.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li BQ, Liang ZY, Seery S, et al. WT1 associated protein promotes metastasis and chemo-resistance to gemcitabine by stabilizing Fak mRNA in pancreatic cancer. Cancer Lett. 2019;451:48-57. doi: 10.1016/j.canlet.2019.02.043 [DOI] [PubMed] [Google Scholar]
- 47.Yu HL, Ma XD, Tong JF, Li JQ, Guan XJ, Yang JH. WTAP is a prognostic marker of high-grade serous ovarian cancer and regulates the progression of ovarian cancer cells. OncoTargets Ther. 2019;12:6191-6201. doi: 10.2147/OTT.S205730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang J, Xu J, Li K, et al. Identification of WTAP-related genes by weighted gene co-expression network analysis in ovarian cancer. J Ovarian Res. 2020;13(1):119. doi: 10.1186/s13048-020-00710-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lyu Y, Zhang Y, Wang Y, et al. HIF-1α regulated WTAP overexpression promoting the Warburg effect of ovarian cancer by m6A-dependent manner. J Immunol Res. 2022;2022:6130806. doi: 10.1155/2022/6130806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fu Y, Jia XC. WTAP-mediated N6-methyladenosine modification on EGR3 in different types of epithelial ovarian cancer. J Biol Regul Homeost Agents. 2020;34(4):1505-1512. doi: 10.23812/20-325-L [DOI] [PubMed] [Google Scholar]
- 51.Gan L, Zhao S, Gao Y, et al. N6-methyladenosine methyltransferase KIAA1429 promoted ovarian cancer aerobic glycolysis and progression through enhancing ENO1 expression. Biol Direct. 2023;18(1):64. doi: 10.1186/s13062-023-00420-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lan Q, Liu PY, Haase J, Bell JL, Hüttelmaier S, Liu T. The critical role of RNA m(6)A methylation in cancer. Cancer Res. 2019;79(7):1285-1292. doi: 10.1158/0008-5472.CAN-18-2965 [DOI] [PubMed] [Google Scholar]
- 53.Liu N, Dai Q, Zheng G, He C, Parisien M, Pan T. N(6)-methyladenosine-dependent RNA structural switches regulate RNA-protein interactions. Nature. 2015;518(7540):560-564. doi: 10.1038/nature14234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang W, Wu T, Zhang Y, et al. Targeting m(6)A binding protein YTHDFs for cancer therapy. Bioorg Med Chem. 2023;90:117373. doi: 10.1016/j.bmc.2023.117373 [DOI] [PubMed] [Google Scholar]
- 55.Liu T, Wei Q, Jin J, et al. The m6A reader YTHDF1 promotes ovarian cancer progression via augmenting EIF3C translation. Nucleic Acids Res. 2020;48(7):3816-3831. doi: 10.1093/nar/gkaa048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hao L, Wang JM, Liu BQ, et al. m6A-YTHDF1-mediated TRIM29 upregulation facilitates the stem cell-like phenotype of cisplatin-resistant ovarian cancer cells. Biochim Biophys Acta Mol Cell Res. 2021;1868(1):118878. doi: 10.1016/j.bbamcr.2020.118878 [DOI] [PubMed] [Google Scholar]
- 57.Xu F, Li J, Ni M, et al. FBW7 suppresses ovarian cancer development by targeting the N(6)-methyladenosine binding protein YTHDF2. Mol Cancer. 2021;20(1):45. doi: 10.1186/s12943-021-01340-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li J, Wu L, Pei M, Zhang Y. YTHDF2, a protein repressed by miR-145, regulates proliferation, apoptosis, and migration in ovarian cancer cells. J Ovarian Res. 2020;13(1):111. doi: 10.1186/s13048-020-00717-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ramesh-Kumar D, Guil S. The IGF2BP family of RNA binding proteins links epitranscriptomics to cancer. Semin Cancer Biol. 2022;86(3):18-31. doi: 10.1016/j.semcancer.2022.05.009 [DOI] [PubMed] [Google Scholar]
- 60.Müller S, Glaß M, Singh AK, et al. IGF2BP1 promotes SRF-dependent transcription in cancer in a m6A- and miRNA-dependent manner. Nucleic Acids Res. 2019;47(1):375-390. doi: 10.1093/nar/gky1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang Y, Chen Z. Long noncoding RNA UBA6-AS1 inhibits the malignancy of ovarian cancer cells via suppressing the decay of UBA6 mRNA. Bioengineered. 2022;13(1):178-189. doi: 10.1080/21655979.2021.2011640 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 62.Sun M, Zhang X, Bi F, et al. FTO inhibits epithelial ovarian cancer progression by destabilising SNAI1 mRNA through IGF2BP2. Cancers. 2022;14(21):5218. doi: 10.3390/cancers14215218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang R, Ye H, Yang B, et al. m6A-modified circNFIX promotes ovarian cancer progression and immune escape via activating IL-6R/JAK1/STAT3 signaling by sponging miR-647. Int Immunopharmacol. 2023;124(A):110879. doi: 10.1016/j.intimp.2023.110879 [DOI] [PubMed] [Google Scholar]
- 64.Yang D, Zhao G, Zhang HM. m(6)A reader proteins: the executive factors in modulating viral replication and host immune response. Front Cell Infect Microbiol. 2023;13:1151069. doi: 10.3389/fcimb.2023.1151069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu Q, Li G, Gong L, et al. Identification of miR-30c-5p as a tumor suppressor by targeting the m(6) A reader HNRNPA2B1 in ovarian cancer. Cancer Med. 2023;12(4):5055-5070. doi: 10.1002/cam4.5246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fang Z, Mei W, Qu C, et al. Role of m6A writers, erasers and readers in cancer. Exp Hematol Oncol. 2022;11(1):45. doi: 10.1186/s40164-022-00298-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dina C, Meyre D, Gallina S, et al. Variation in FTO contributes to childhood obesity and severe adult obesity. Nat Genet. 2007;39(6):724-726. doi: 10.1038/ng2048 [DOI] [PubMed] [Google Scholar]
- 68.Zhang QC, Qian YM, Ren YH, et al. Phenethyl isothiocyanate inhibits metastasis potential of non-small cell lung cancer cells through FTO mediated TLE1 m(6)A modification. Acta Pharmacol Sin. 2023;45(3):619-632. doi: 10.1038/s41401-023-01178-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li Z, Weng H, Su R, et al. FTO plays an oncogenic role in acute myeloid leukemia as a N(6)-Methyladenosine RNA demethylase. Cancer Cell. 2017;31(1):127-141. doi: 10.1016/j.ccell.2016.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lin K, Zhou E, Shi T, et al. m6A eraser FTO impairs gemcitabine resistance in pancreatic cancer through influencing NEDD4 mRNA stability by regulating the PTEN/PI3K/AKT pathway. J Exp Clin Cancer Res. 2023;42(1):217. doi: 10.1186/s13046-023-02792-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huang H, Wang Y, Kandpal M, et al. FTO-dependent N (6)-methyladenosine modifications inhibit ovarian cancer stem cell self-renewal by blocking cAMP signaling. Cancer Res. 2020;80(16):3200-3214. doi: 10.1158/0008-5472.CAN-19-4044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang Z, Zhu H, Hu J. CircRAB11FIP1 promoted autophagy flux of ovarian cancer through DSC1 and miR-129. Cell Death Dis. 2021;12(2):219. doi: 10.1038/s41419-021-03486-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang H, Zhao G, Cardenas H, Valdivia AF, Wang Y, Matei D. N6-methyladenosine RNA modifications regulate the response to platinum through nicotinamide N-methyltransferase. Mol Cancer Ther. 2023;22(3):393-405. doi: 10.1158/1535-7163.MCT-22-0278 [DOI] [PubMed] [Google Scholar]
- 74.Chen L, Gao W, Lin L, et al. A methylation- and immune-related lncRNA signature to predict ovarian cancer outcome and uncover mechanisms of chemoresistance. J Ovarian Res. 2023;16(1):186. doi: 10.1186/s13048-023-01260-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang J, Wang J, Gu Q, et al. The biological function of m6A demethylase ALKBH5 and its role in human disease. Cancer Cell Int. 2020;20:347. doi: 10.1186/s12935-020-01450-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhu H, Gan X, Jiang X, Diao S, Wu H, Hu J. ALKBH5 inhibited autophagy of epithelial ovarian cancer through miR-7 and BCL-2. J Exp Clin Cancer Res. 2019;38(1):163. doi: 10.1186/s13046-019-1159-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fukumoto T, Zhu H, Nacarelli T, et al. N(6)-methylation of adenosine of FZD10 mRNA contributes to PARP inhibitor resistance. Cancer Res. 2019;79(11):2812-2820. doi: 10.1158/0008-5472.CAN-18-3592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nie S, Zhang L, Liu J, et al. ALKBH5-HOXA10 loop-mediated JAK2 m6A demethylation and cisplatin resistance in epithelial ovarian cancer. J Exp Clin Cancer Res. 2021;40(1):284. doi: 10.1186/s13046-021-02088-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sun R, Yuan L, Jiang Y, et al. ALKBH5 activates FAK signaling through m6A demethylation in ITGB1 mRNA and enhances tumor-associated lymphangiogenesis and lymph node metastasis in ovarian cancer. Theranostics. 2023;13(2):833-848. doi: 10.7150/thno.77441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jiang Y, Wan Y, Gong M, Zhou S, Qiu J, Cheng W. RNA demethylase ALKBH5 promotes ovarian carcinogenesis in a simulated tumour microenvironment through stimulating NF-κB pathway. J Cell Mol Med. 2020;24(11):6137-6148. doi: 10.1111/jcmm.15228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fan L, Lin Y, Lei H, et al. A newly defined risk signature, consisting of three m(6)A RNA methylation regulators, predicts the prognosis of ovarian cancer. Aging. 2020;12(18):18453-18475. doi: 10.18632/aging.103811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li Q, Ren CC, Chen YN, et al. A risk score model incorporating three m6A RNA methylation regulators and a related network of miRNAs-m6A regulators-m6A target genes to predict the prognosis of patients with ovarian cancer. Front Cell Dev Biol. 2021;9:703969. doi: 10.3389/fcell.2021.703969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang C, Liu J, Guo H, et al. m6A RNA methylation regulators were associated with the malignancy and prognosis of ovarian cancer. Bioengineered. 2021;12(1):3159-3176. doi: 10.1080/21655979.2021.1946305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Han X, Liu J, Cheng G, Cui S. Gene signatures and prognostic values of m6A RNA methylation regulators in ovarian cancer. Cancer Control J Moffitt Cancer Cent. 2020;27(1):1073274820960460. doi: 10.1177/1073274820960460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang Q, Zhang Q, Li Q, Zhang J, Zhang J. Clinicopathological and immunological characterization of RNA m6 A methylation regulators in ovarian cancer. Mol Genet Genomic Med. 2021;9(1):e1547. doi: 10.1002/mgg3.1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tan W, Liu S, Deng Z, et al. Gene signature of m6A-related targets to predict prognosis and immunotherapy response in ovarian cancer. J Cancer Res Clin Oncol. 2023;149(2):593-608. doi: 10.1007/s00432-022-04162-3 [DOI] [PubMed] [Google Scholar]
- 87.Zhu W, Zhao L, Kong B, et al. The methylation modification of m6A regulators contributes to the prognosis of ovarian cancer. Ann Transl Med. 2022;10(2):59. doi: 10.21037/atm-21-6462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yankova E, Blackaby W, Albertella M, et al. Small-molecule inhibition of METTL3 as a strategy against myeloid leukaemia. Nature. 2021;593(7860):597-601. doi: 10.1038/s41586-021-03536-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cully M. Chemical inhibitors make their RNA epigenetic mark. Nat Rev Drug Discov. 2019;18(12):892-894. doi: 10.1038/d41573-019-00179-5 [DOI] [PubMed] [Google Scholar]
- 90.Pan Y, Chen H, Zhang X, et al. METTL3 drives NAFLD-related hepatocellular carcinoma and is a therapeutic target for boosting immunotherapy. Cell Rep Med. 2023;4(8):101144. doi: 10.1016/j.xcrm.2023.101144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bedi RK, Huang D, Eberle SA, Wiedmer L, Śledź P, Caflisch A. Small-molecule inhibitors of METTL3, the major human epitranscriptomic writer. ChemMedChem. 2020;15(9):744-748. doi: 10.1002/cmdc.202000011 [DOI] [PubMed] [Google Scholar]
- 92.Moroz-Omori EV, Huang D, Kumar Bedi R, et al. METTL3 inhibitors for epitranscriptomic modulation of cellular processes. ChemMedChem. 2021;16(19):3035-3043. doi: 10.1002/cmdc.202100291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ding N, You A, Tian W, Gu L, Deng D. Chidamide increases the sensitivity of non-small Cell Lung Cancer to crizotinib by decreasing c-MET mRNA methylation. Int J Biol Sci. 2020;16(14):2595-2611. doi: 10.7150/ijbs.45886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zou Z, Wei J, Chen Y, et al. FMRP phosphorylation modulates neuronal translation through YTHDF1. Mol Cell. 2023;83(23):4304-4317.e8. doi: 10.1016/j.molcel.2023.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Weng H, Huang F, Yu Z, et al. The m(6)A reader IGF2BP2 regulates glutamine metabolism and represents a therapeutic target in acute myeloid leukemia. Cancer Cell. 2022;40(12):1566-1582.e10. doi: 10.1016/j.ccell.2022.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shu C, Gu MH, Zeng C, et al. Small-molecule exhibits anti-tumor activity by targeting the RNA m(6)A reader IGF2BP3 in ovarian cancer. Am J Cancer Res. 2023;13(10):4888-4902. [PMC free article] [PubMed] [Google Scholar]
- 97.Huang Y, Yan J, Li Q, et al. Meclofenamic acid selectively inhibits FTO demethylation of m6A over ALKBH5. Nucleic Acids Res. 2015;43(1):373-384. doi: 10.1093/nar/gku1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xiao L, Li X, Mu Z, et al. FTO inhibition enhances the antitumor effect of temozolomide by targeting MYC-miR-155/23a cluster-MXI1 feedback circuit in glioma. Cancer Res. 2020;80(18):3945-3958. doi: 10.1158/0008-5472.CAN-20-0132 [DOI] [PubMed] [Google Scholar]
- 99.Liu J, Song Y, Wang Y, Han M, Wang C, Yan F. Cyclodextrin-functionalized gold nanorods loaded with meclofenamic acid for improving N(6)-Methyladenosine-Mediated second near-infrared photothermal immunotherapy. ACS Appl Mater Interfaces. 2022;14(36):40612-40623. doi: 10.1021/acsami.2c09978 [DOI] [PubMed] [Google Scholar]
- 100.Huang Y, Su R, Sheng Y, et al. Small-molecule targeting of oncogenic FTO demethylase in acute myeloid leukemia. Cancer Cell. 2019;35(4):677-691.e10. doi: 10.1016/j.ccell.2019.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xiao Z, Li T, Zheng X, et al. Nanodrug enhances post-ablation immunotherapy of hepatocellular carcinoma via promoting dendritic cell maturation and antigen presentation. Bioact Mater. 2023;21:57-68. doi: 10.1016/j.bioactmat.2022.07.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Su R, Dong L, Li Y, et al. Targeting FTO suppresses cancer stem cell maintenance and immune evasion. Cancer Cell. 2020;38(1):79-96.e11. doi: 10.1016/j.ccell.2020.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wu R, Yao Y, Jiang Q, et al. Epigallocatechin gallate targets FTO and inhibits adipogenesis in an mRNA m(6)A-YTHDF2-dependent manner. Int J Obes (Lond). 2018;42(7):1378-1388. doi: 10.1038/s41366-018-0082-5 [DOI] [PubMed] [Google Scholar]
- 104.Huff S, Tiwari SK, Gonzalez GM, Wang Y, Rana TM. m(6)A-RNA demethylase FTO inhibitors impair self-renewal in glioblastoma stem cells. ACS Chem Biol. 2021;16(2):324-333. doi: 10.1021/acschembio.0c00841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu Y, Liang G, Xu H, et al. Tumors exploit FTO-mediated regulation of glycolytic metabolism to evade immune surveillance. Cell Metab. 2021;33(6):1221-1233.e11. doi: 10.1016/j.cmet.2021.04.001 [DOI] [PubMed] [Google Scholar]
- 106.Malacrida A, Rivara M, Di Domizio A, et al. 3D proteome-wide scale screening and activity evaluation of a new ALKBH5 inhibitor in U87 glioblastoma cell line. Bioorg Med Chem. 2020;28(4):115300. doi: 10.1016/j.bmc.2019.115300 [DOI] [PubMed] [Google Scholar]
- 107.Li N, Kang Y, Wang L, et al. ALKBH5 regulates anti-PD-1 therapy response by modulating lactate and suppressive immune cell accumulation in tumor microenvironment. Proc Natl Acad Sci U S A. 2020;117(33):20159-20170. doi: 10.1073/pnas.1918986117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]