Abstract
Objective
Rheumatoid arthritis (RA) is a chronic inflammatory and destructive arthritis, characterized by inflammatory infiltration and bone destruction. Huangqi Guizhi Wuwu Decoction (HGWD) is traditional Chinese medicine, which has been applied in the treatment of RA in clinical. The aim of this study was to investigate the therapeutic effect of HGWD on collagen-induced arthritis (CIA) mouse model.
Methods
DBA/1J female mice were used to establish the collagen-induced arthritis (CIA) model. HGWD was administered intragastrically once a day for four weeks starting on the 22nd day after the first immunization. The body weight, hind paw thickness and clinical score were measured every five days. Gait analysis, histopathological staining, enzyme-linked immunosorbent assay (ELISA), ultrasound imaging and micro-computed tomography imaging were performed to determine the effects of HGWD treatment on inflammation and bone structure in this model. Moreover, Real-time PCR and Western blot analysis were used to detect inflammatory factors mRNA and protein levels after HGWD intervention in RAW 264.7 cells.
Results
HGWD attenuated symptoms of arthritis, suppressed inflammatory synovium area and the serum levels of inflammatory factors, inhibited joint space enlargement in the knee and ankle joints, reduced numbers of osteoclasts, protected bone destruction, as well as improved motor function. HGWD decreased the expression of mRNA for inflammatory factors and the protein expression levels of p-NF-кB and IL-17.
Conclusion
These results suggested that HGWD suppresses inflammation, attenuates bone erosion and maintains motor function in collagen-induced arthritis mice.
Keywords: bone destruction, collagen-induced arthritis, Huangqi Guizhi Wuwu Decoction, inflammation, rheumatoid arthritis
1. Introduction
A chronic inflammatory autoimmune condition known as rheumatoid arthritis (RA), which is characterized by synovial hyperplasia, inflammatory infiltration, subsequent cartilage destruction and bone erosion (Genovese et al., 2019, Klareskog et al., 2009). These changes could lead to joint deformity, disability, and low quality of life. There are nearly five million people living with RA in China, representing a significant social problem (Jin et al., 2017, Xie et al., 2019).
In clinic, RA is mainly treated by disease-modifying anti-rheumatic drugs (DMARDs), such as methotrexate (MTX), leflunomide and hydroxychloroquine. MTX is the most commonly used DMARDs. The DMARDs may lessen symptoms and enhance the quality of life in addition to limiting joint deterioration and maintaining joint structure and functioning (Yap et al., 2018, He et al., 2021, He et al., 2021). However, long-term usage of these medications does have a number of negative effects, including but not limited to stomach upset, diarrhea, interstitial pneumonitis and liver damage (Kalashnikova et al., 2020).
According to traditional Chinese medical philosophy, RA is classified as Bi syndrome, which is mainly caused by wind-cold-wetness evil. The treatment of RA with traditional Chinese medicine (TCM) has a long history. Meanwhile, TCM is less expensive, effective, safe, and with fewer side effects.
Huangqi Guizhi Wuwu Decoction (HGWD), a classical prescription of TCM, was derived from Synopsis of the Golden Chamber (Jingui Yaolue in Chinese) by Zhongjing Zhang in Eastern Han Dynasty. This formula includes five herbs, Hedysarum multijugum Maxim. (Honghuayan Huangqi in Chinese), Cinnamomi Ramulus (Guizhi in Chinese), Paeoniae Radix Alba (Baishao in Chinese), Zingiberis Rhizoma Recens (Shengjiang in Chinese), and Jujubae Fructus (Dazao in Chinese). HGWD has effects of invigorating qi and warming meridians, activating blood and releasing “Bi”, dispersing dampness and draining cold (Chen et al., 2015, Tang et al., 2023). It is also used to anti-inflammation and enhancing body immunity (Liu et al., 2019). HGWD could be used to treat RA in clinic (Wang et al., 2020). However, how HGWD affects RA has not been fully understood. Therefore, this study was designed to investigate the treatment effects of HGWD on inflammation and bone destruction in the collagen-induced arthritis (CIA) mouse model.
2. Materials and methods
2.1. Preparation of Huangqi Guizhi Wuwu Decoction
The composition of HGWD is H. multijugum 9 g, Cinnamomi Ramulus 9 g, Paeoniae Radix Alba 9 g, Zingiberis Rhizoma Recens 18 g, and Jujubae Fructus 15 g. All herbs were obtained from the Chinese Pharmacy of Longhua Hospital, which is affiliated with the Shanghai University of Traditional Chinese Medicine. The method of preparing extracts of HGWD and HPLC quality was as previously published article (Wang et al., 2022), and the results were shown in Fig. S1 (supplementary materials).
2.2. Animals
A total of 32 specific pathogen-free DBA/1J female mice with 6–8 weeks old (Animal Certificate of Conformity license number: SCXK(Hu)2014–0042) were used in this experiment. They were obtained and kept at the Shanghai Model Organisms Center (Shanghai, China). The Shanghai Model Organisms Center's Institutional Animal Care and Use Committee examined and approved the animal research.
2.3. Collagen-induced arthritis (CIA) model
The CIA model was created according to the methods previously described (Brand, Latham, & Rosloniec, 2007). At 4 °C overnight, Bovine Collagen Type II (Chondrex Inc, Cat No. #20021) was dissolved in 10 mmol/L acetic acid to 2 mg/mL. Then, this solution was emulsified with an equivalent amount of complete Freund’s adjuvant (Chondrex Inc, Cat No. #7001). The mice were given 100 μL of emulsion intradermally at the base of the tail (day 0, first immunization). The mice were immunized again on day 21 with bovine collagen type II emulsified in incomplete Freund’s adjuvant (Chondrex Inc, Cat No. #7002). In this model, the first sign of arthritis appears between 21 and 28 days after the first immunization.
2.4. Groups and treatments
A total of 32 mice were randomly distributed into four groups with eight animals in each group. The mice without CIA induction were used as a normal control group (control). On the 22nd day after the first immunization, the mice which accepted CIA were given 0.2 mL of saline orally and daily as a model group (CIA + saline) or 0.2 mL of MTX (0.075 mg/mL, Shanghai Oriental Medicine Science and Technology Industry Co., Ltd., Cat No. #100138) intragastrically twice a week as a positive control group (CIA + MTX) or 0.2 mL of HGWD (0.8 mg/kg) by gastric gavage once a day as a HGWD treatment group (CIA + HGWD) for four weeks.
2.5. Arthritis assessment
Twenty-one days after the first immunization, we measured the body weight, hind paw thickness and clinical score every five days. Clinical score was based on visual observation, each paw was scored 0–4 (Wang et al., 2018, Yabe et al., 2021). The following scores were assigned: 0, no swelling (normal); 1, erythema and minor swelling restricted to individual digits; 2, erythema and mild swelling in multiple digits; 3, erythema and moderate swelling; and 4, erythema and severe swelling. Each mouse's ultimate score was the total of the scores from all four paws. The clinical score was assessed by two independent examiners blinded to the whole group.
2.6. Gait analysis
Gait analysis was collected using the GaitScan system (Clever Sys Inc, Washington, USA). Footprints were captured using a high-speed camera and Bcam Cap Image Capture. These recorded videos were analyzed using TreadScan TM 2.0 software (Clever Sys Inc, Washington, USA). Each mouse was tested three times. The data were shown as the mean from three tests from each mouse.
2.7. Ultrasound imaging and analysis
The Vevo 2100 imaging system (FUJIFILM Visual Sonics Inc, Toronto, Canada) was used to check the knee and ankle joints. They were scanned using B-mode according to the methods previously described (Jia et al., 2019, Xu et al., 2017). Three-dimensional images were reconstructed and quantified from B-mode images using VevoLab software (FUJIFILM Visual Sonics Inc, Toronto, Canada).
2.8. Histology
Left ankle and knee joints were fixed in 4% paraformaldehyde solution for 24 h, decalcified in 10% sodium ethylene diamine tetraacetic acid (EDTA) for 21 d, embedded in paraffin and sectioned into 4 μm/slide. Alcian Blue/Orange G (ABOG) staining and tartrate-resistant acid phosphatase (TRAP) staining were used to observe histopathological changes.
2.9. Enzyme-linked immunosorbent assay (ELISA)
Serum samples were extracted from peripheral blood and stored at −80 °C. The ELISA kit of TNF-α, IL-1β and IL-6 were all from Elabscience Biotechnology (Elabscience Biotechnology Inc, Wuhan, China. Cat No. #E-EL-M0049c, E-EL-M0037c and E-EL-M0044c). All operations were carried out in accordance with the manufacturer's instructions.
2.10. Micro-Computed tomography (Micro-CT) image
Right ankle joints were fixed in 4% paraformaldehyde solution for 24 h, then rinsed under running water for 2 h before being kept in 75% ethanol and scanned by micro-CT system (Scanco viva CT80, Scanco Medical AG, Brüttisellen, Switzerland). Here are the settings: tube voltage 55 kV, tube current 72 μA, exposure time 200 ms. Scanco's supporting software was then used to rebuild and evaluate three-dimensional pictures (Scanco Medical AG, Brüttisellen, Switzerland).
2.11. Cell line and culture conditions
Murine macrophage (RAW264.7) cells were obtained from the research group, which grown using DMEM (HyClone, Cat. No. SH30022.01), supplemented with 10% fetal bovine serum (Gibco, New York, USA. Cat. No. 10270–106) and added 1% penicillin/streptomycin (Biological Industries, Cat. No. 03–031-1B). RAW264.7 cells were cultured at 37 °C and 5% CO2.
2.12. Cell viability assay
Viability of RAW264.7 was assessed by Cell Counting Kit-8 (CCK-8) assay. RAW264.7 cells were seeded in 96-well culture plates at 1 × 104 cells/well. After 24 h, RAW264.7 treated with different concentrations of HGWD (0, 0.25, 0.5, 1, 1.5 and 2 mg/mL) for another 24 h. Then, CCK-8 reagent (Dojindo, Kyushu Island, Japan. Cat. No. NQ643) was added into each well and mixed fully at 37 °C for 1 h. The absorbance was detected at 450 nm with a microplate reader (BioTek Synergy 3, Vermont).
2.13. Real-time PCR
Total RNA from RAW264.7 was isolated using EZ-press RNA Purification Kit (EZBioscience, Minnesota, USA. Cat. No. B0004D). The cDNA was prepared using the Color Reverse Transcription Kit with gDNA Remover (EZBioscience, Cat. No. A0010GQ), and real-time PCR was performed using 2 × SYBR Green qPCR Master Mix (ROX2 plus) (EZBioscience, Cat. No. A0001-R2). The relative mRNA levels of the target genes were determined using the 2−ΔΔCt method. This experiment was repeated three times.
2.14. Western blot
The RAW264.7 was collected, and the total protein was collected. The protein was separated by 10% SDS-PAGE and transferred to PVDF membranes. Western blot was performed using rabbit anti-p-NF-кB antibody (1:1 000; Cell Signaling Technology, Boston, USA. Cat. No. 3033), anti-NF-кB antibody (1:1 000; Cell Signaling Technology, Cat. No. 8242), rabbit anti-IL-17 antibody (1:1 000; Abcam, Cat. No. ab79056), and mouse anti-β-actin antibody (1:2 000; Sigma, Missouri, USA. Cat. No. A2228). The protein bands were visualized using ECL (Millipore, Massachusetts, USA. Cat. No. WBKLS0500) and chemiluminescence detection system (Bio-Rad, California, USA).
2.15. Statistical analysis
The data were shown as the mean values ± standard deviation. GraphPad Prism 8.0.2 (GraphPad Software, La Jolla, CA) was used for statistical analyses and representation. Differences between multiple groups were compared using One-way analysis of variance (ANOVA) with Tukey’s multiple comparison test. The two-way ANOVA comparison was done for multiple groups with different time points. A statistically significant difference was defined as P < 0.05.
3. Results
3.1. HGWD attenuated symptoms of CIA mice
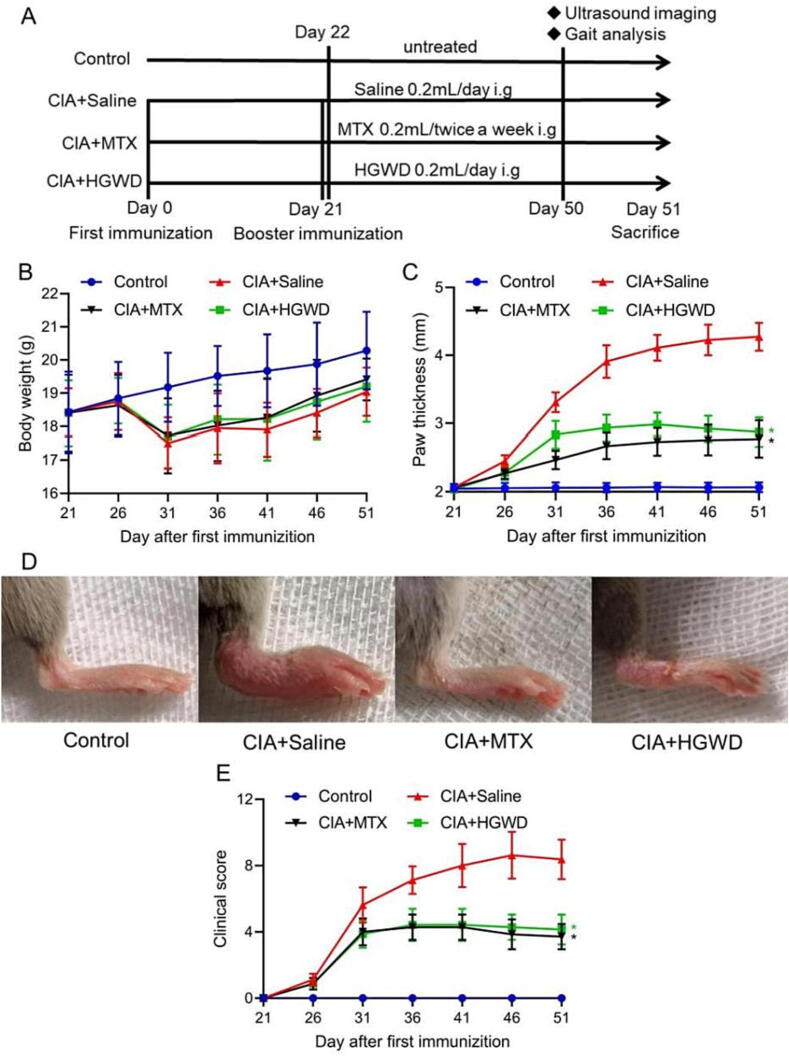
We employed a CIA model in DBA/1J mice to study the effects of HGWD on RA (Fig. 1A). When compared to the control group, the CIA model mice exhibited a modest drop in body weight from day 26 to day 31, followed by an increase (Fig. 1B). In this model, mice develop arthritic symptoms including varying degrees of swelling of the toes, soles and ankle joints after booster immunization (Day 21). After four weeks treatment, the hind paw thickness and clinical score were significantly decreased in the MTX-treated and HGWD-treated group compared with the saline-treated group (Fig. 1C−E). These results suggested that HGWD can effectively inhibit the symptoms of arthritis in this model.
Fig. 1.
HGWD attenuated symptoms of CIA (mean ± SD, n = 8). (A) Schedule of experimental groups and drug administration. Body weight (B), paw thickness (C) and clinical score (E) in each group over time. (D) Representative pictures of hind paws of each group on day 51. *P < 0.05 vs CIA + Saline group.
3.2. HGWD improved motor function of CIA mice
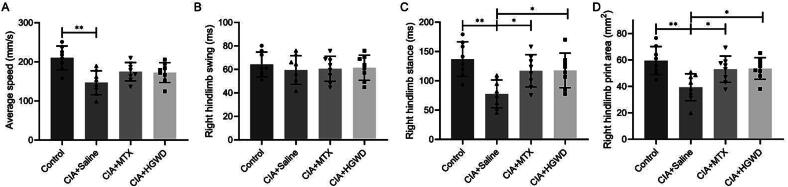
Gait analysis revealed that the average speed of the model group was lower than that of the control group (Fig. 2A). Groups did not differ in swing time (Fig. 2B). The model and control groups varied in stance time and print area in the right hind limbs. And therapy may be able to reverse some of these alterations (Fig. 2C and D). These combined results indicate that MTX or HGWD treatment improves motor function in mice with the CIA model.
Fig. 2.
HGWD improved gait function (mean ± SD, n = 8). Gait parameters of average speed (A), right hindlimb swing (B), right hindlimb stance (C), and right hindlimb print area (D) in each group at day 51. *P < 0.05, **P < 0.01 vs CIA + Saline group.
3.3. HGWD inhibited joint space enlargement in knee and ankle joint of CIA mice
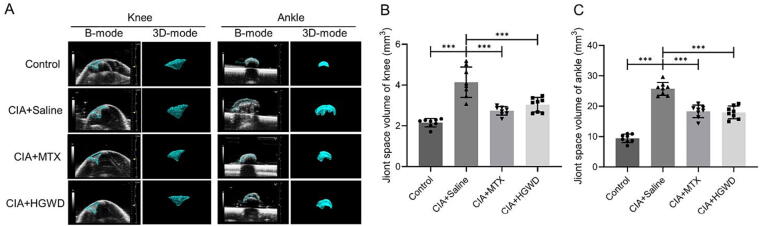
The Vevo 2100 imaging system was used to evaluate the volume of knee and ankle joint cavities in mice after treatment. The results of ultrasound imaging and analysis showed that CIA mice joint space enlarged. And HGWD-treated group performed similar therapeutic effect on significantly inhibiting joint space enlargement in the knee and ankle joints of the CIA mice compared to the MTX-treated group (Fig. 3).
Fig. 3.
HGWD inhibited joint space enlargement in knee and ankle joint of CIA mice. (A) Representative images of B mode and three-dimensional reconstructions of knee and ankle joints. Quantification of joint space volume of knee (B) and ankle (C) (mean ± SD, n = 8). ***P < 0.001 vs CIA + Saline group.
3.4. HGWD reduces inflammation of CIA mice
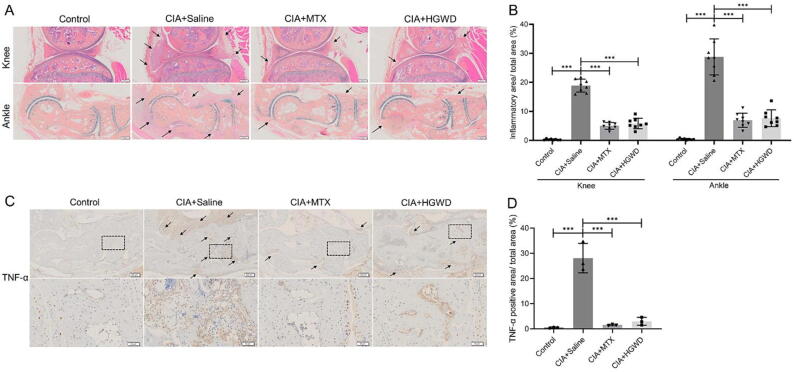
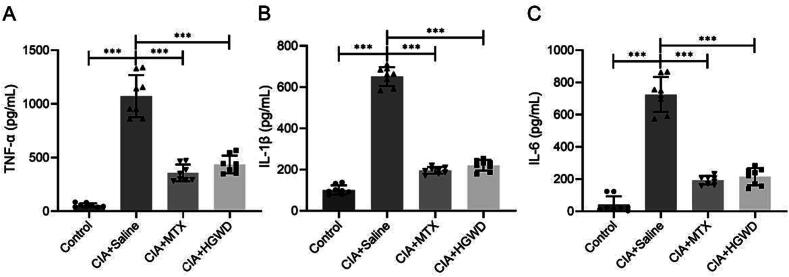
To observe the effect of HGWD on the histopathology of the CIA model, we performed ABOG staining on the knee and ankle joints and quantified the area of inflammation. According to representative images of ABOG staining (Fig. 4A), we discovered that saline-treated knee and ankle joints showed severe inflammatory infiltration, while MTX-treated and HGWD-treated knee and ankle joints had dramatically decreased inflammatory synovium area (Fig. 4B). There is a large amount of TNF-α expression around the ankle joint in the saline group, and TNF-α expression was significantly reduced in the MTX-treated and HGWD-treated group. (Fig. 4C and D). In addition, we collected the serum of the mice and detected the level of serum inflammatory factors by ELISA kit. According to the findings, the serum TNF-α, IL-1β and IL-6 levels were considerably lower in the treated group than in the saline group (Fig. 5).
Fig. 4.
HGWD reduces inflammation of knee and ankle joint. (A) Representative images of ABOG staining of knee and ankle joint (scale bar = 200 μm). (B) Quantification of inflammatory area/ total area (mean ± SD, n = 8). (C) Representative images of TNF-α immunohistochemical staining of ankle joint. Scale bar = 200 μm in upper pictures; Scale bar = 50 μm in bottom pictures. (D) Quantification of TNF-α positive area/ total area (mean ± SD, n = 3). ***P < 0.001 vs CIA + Saline group.
Fig. 5.
HGWD reduces levels of inflammatory factors of TNF-α (A), IL-1β (B), and IL-6 (C) in serum (mean ± SD, n = 8). ***P < 0.001 vs CIA + Saline group.
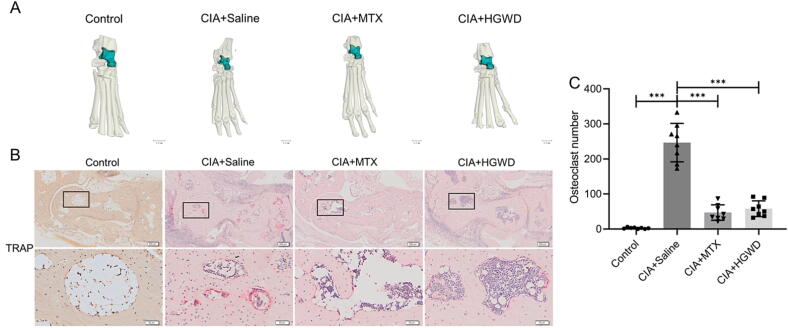
3.5. HGWD protected bone destruction of CIA mice
Three-dimensional reconstruction micro-CT images showed that the talus, scaphoid, cuneiform and metatarsal bones of CIA mice had severe bone erosion, while the degree of bone erosion in the MTX group and the HGWD group were reduced compared with the model group, and the bone structures in the two groups were relatively complete (Fig. 6A). Furthermore, we performed TRAP staining to assess bone destruction by osteoclasts. It was found that the drug-treated group had fewer osteoclasts surrounding the astragalus in the ankle joints than the saline-treated group, according to TRAP staining (Fig. 6B and C).
Fig. 6.
HGWD protected bone destruction. (A) Representative 3D micro-CT images of ankle joints (scale bar = 1 mm). (B) Representative TRAP staining images. Scale bar = 200 μm in upper pictures; Scale bar = 50 μm in bottom pictures. (C) Quantification of number of TRAP-positive osteoclasts (mean ± SD, n = 8). ***P < 0.001 vs CIA + Saline group.
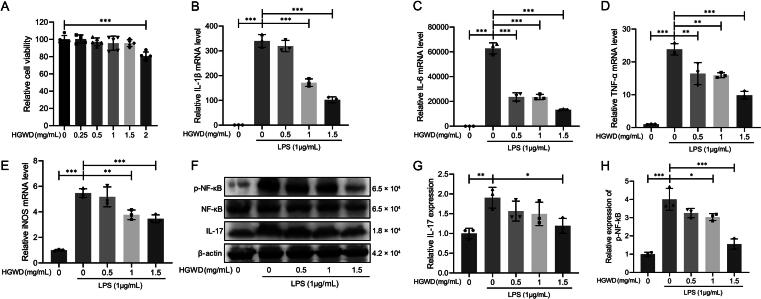
3.6. HGWD suppressed mRNA and protein expression of inflammatory factors in LPS-induced Raw264.7 cells
To further investigate the mechanism of HGWD action, we examined its effect on LPS-induced Raw264.7 cells. Raw264.7 cells were pretreated with the different concentrations of HGWD, prior to stimulation with LPS (1 μg/mL). Then, the inflammatory factors mRNA levels and the protein levels of p-NF-кB, NF-кB and IL-17 were determined. We found that 0–1.5 mg/mL HGWD did not affect cell viability, 2 mg/mL HGWD inhibited cell viability (Fig. 7A). HGWD markedly decreased the expression of mRNA for IL-1β, IL-6, TNF-α and iNOS (Fig. 7B−E). And HGWD treatment significantly suppressed the protein expression levels of p-NF-кB and IL-17 (Fig. 7F−H). These changes were dose dependent.
Fig. 7.
HGWD suppressed mRNA and protein expression of inflammatory factors. (A) Cell viability of Raw264.7 treated by different concentrations of HGWD for 24 h (mean ± SD, n = 5). (B−E) mRNA levels of IL-1β, IL-6, TNF-α and iNOS in Raw264.7 (mean ± SD, n = 3). (F) Representative WB image. Quantitative analysis of IL-17 (G) and p-NF-кB (H) protein expression (mean ± SD, n = 3). *P < 0.05, **P < 0.01, ***P < 0.001 vs CIA + Saline group.
4. Discussion
HGWD, a classic TCM prescription, has been utilized in therapeutic settings in China for thousands of years (Liu et al., 2021). The H. multijugum promotes blood circulation, removes blood stasis and replenishes yang (Li, Cao, Yao, & Lei, 2021). Cinnamomi Ramulus has the functions of replenishing spleen and kidney yang to driving away cold and transporting water (Kim et al., 2016). Paeoniae Radix Alba could nourish yin and blood (Duan, Pan, Bao, & Peng, 2019). HGWD has been used in the treatment of blood stasis, such as RA, numbness sensory disturbance, cervical spondylosis, heart disease, and stroke (He et al., 2021, He et al., 2021, Pang et al., 2016). Nowadays, it is becoming increasingly popular to use TCM to prevent or treat chronic disease, due to the benefits of minor toxicity and few side effects (Wei et al., 2020). MTX is known to be effective slowing the progression of joint damage in RA patients, but it also has some adverse effects such as liver damage, gastrointestinal symptoms, and bone marrow suppression (Zhai et al., 2021). There are some clinical studies have revealed that HGWD coupled with western medicine has superior treatment effects on RA patients than the western medicine control group (Ma, Ma, & Zhao, 2016). However, there are only a few animal experiments exploring treatment for RA model. Against this background, we adopted CIA mouse models to verify the effect of HGWD on RA. In this study, we demonstrated that HGWD could suppress symptoms of arthritis and preserve motor function through reducing inflammation and bone destruction.
RA is a common chronic inflammatory disorder, and has more synovial fluid, consisting of a large number of cytokines around joint (Kim et al., 2018). Excessive synovial fluid stimulates inflammatory cells to produce the inflammatory factors, causing pain and swelling in the joints (McInnes and Schett, 2011, Szekanecz et al., 1994). Meanwhile, inflammatory cells secrete many cytokines, such as matrix metalloproteinases (MMPs) and adhesion molecules, which play important role in the destruction of bone, further leading to loss of function (Lee et al., 2012, Li et al., 2015, Li et al., 2018). Hence, anti-inflammatory therapy is always the preferred treatment option for RA patients in clinic (Ten Klooster, de Graaf, & Vonkeman, 2019). Some previous study reported that HGWD could decrease the expression of serum inflammatory factors TNF-α, IL-1β in adjuvant arthritis rats (Xu, Lin, & Wang, 2007), as well as serum cytokines such as TNF-α, IL-1β, IL-2, IL-3, IL-6, IL-10, and IL-20 in CIA rats (Lin et al., 2022, Liu et al., 2017, Liu et al., 2019, Yang and Feng, 2012). Network pharmacology analysis showed that TNF and IL-17 signaling pathways were identified as key HGWD pathways in the treatment of RA (Liu et al., 2020). Similar to those results, in this study, we determined that HGWD markedly decreased the level of serum TNF-α, IL-1β, IL-6, and diminished inflammatory area around knee and ankle joints. Additionally, HGWD also significantly decreased the expression of mRNA for inflammatory factors, including IL-1β, IL-6, TNF-α and iNOS, and suppressed the protein expression levels of p-NF-кB and IL-17. When the inflammation reduced, paw swelling and joint space were obviously decreased. Apart from these, we also found that HGWD protected against bone erosion. Moreover, gait analysis displayed that HGWD improves CIA mice motor function in average speed, stance time and print area. These results may be due to pain relief by HGWD treatment.
5. Conclusion
In conclusion, we confirmed for the first time that HGWD has therapeutic influences on the CIA mice model. HGWD could markedly alleviate arthritis symptoms, suppress inflammation, attenuate bone erosion and maintain motor function. And the mechanism of HGWD may be through inhibition of the NF-кB/IL-17 signaling pathways. This study provides a scientific basis for the HGWD in the treatment of RA.
CRediT authorship contribution statement
Jiamin Bao: Data curation, Formal analysis, Writing – original draft. Yongjia Song: Data curation, Formal analysis, Writing – original draft. Minghui Hang: Data curation, Formal analysis. Hao Xu: Supervision. Qiang Li: Methodology. Pengyu Wang: Formal analysis. Tao Chen: Methodology, Formal analysis. Mengxiong Xia: Methodology. Qi Shi: Supervision. Yongjun Wang: Funding acquisition, Writing – review & editing. Xiaoyun Wang: Funding acquisition, Writing – original draft. Qianqian Liang: Funding acquisition, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was sponsored by National Natural Science Foundation (No. 81822050, 81920108032, 81904227), Shanghai “Science and Technology Innovation Action Plan” Medical Innovation Research Project (No. 21Y11921400), the Program for Innovative Research Team of Ministry of Science and Technology of China (No. 2015RA4002), “Innovation Team” Development Projects (No. IRT1270), Innovative Team Project of Scientific Research Project of Traditional Chinese Medicine of Shanghai Municipal Health Commission (No. 2022CX001), Shanghai TCM Medical Center of Chronic Disease (No. 2022ZZ01009), Jing'an District Health Research Project of Shanghai (No. 2022MS03).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.chmed.2023.10.003.
Contributor Information
Xiaoyun Wang, Email: 782681050@qq.com.
Qianqian Liang, Email: liangqianqian@shutcm.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Brand D.D., Latham K.A., Rosloniec E.F. Collagen-induced arthritis. Nature Protocols. 2007;2(5):1269–1275. doi: 10.1038/nprot.2007.173. [DOI] [PubMed] [Google Scholar]
- Chen B., Yuan P.W., Kang W.L., Zhang X.L., Liu D.Y. Application of Huangqi Guizhi Wuwu Decoction in orthopedics. Chinese Journal of Traditional Medical Traumatology & Orthopedics. 2015;23(5):71–74. [Google Scholar]
- Duan X., Pan L., Bao Q., Peng D. UPLC-Q-TOF-MS study of the mechanism of THSWD for breast cancer treatment. Frontiers in Pharmacology. 2019;10:1625. doi: 10.3389/fphar.2019.01625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese M.C., Kalunian K., Gottenberg J.E., Mozaffarian N., Bartok B., Matzkies F., Gao J., Guo Y., Tasset C., Sundy J.S., de V., Walker D., Takeuchi T. Effect of filgotinib vs placebo on clinical response in patients with moderate to severe rheumatoid arthritis refractory to disease-modifying antirheumatic drug therapy: The finch 2 randomized clinical trial. Journal of the American Medical Association. 2019;322(4):315–325. doi: 10.1001/jama.2019.9055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y., Zheng H., Zhong L., Zhong N., Wen G., Wang L., Zhang Y. Identification of active ingredients of Huangqi Guizhi Wuwu Decoction for promoting nerve function recovery after ischemic stroke using HT22 live-cell-based affinity chromatography combined with HPLC-MS/MS. Drug Design, Development and Therapy. 2021;15:5165–5178. doi: 10.2147/DDDT.S333418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y.F., Mai C.T., Pan H.D., Liu L., Zhou H., Xie Y. Targeting immunometabolism by active ingredients derived from traditional Chinese medicines for treatment of rheumatoid arthritis. Chinese Herbal Medicines. 2021;13(4):451–460. doi: 10.1016/j.chmed.2021.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Q., Wang T., Wang X., Xu H., Liu Y., Wang Y., Shi Q., Liang Q. Astragalin suppresses inflammatory responses and bone destruction in mice with collagen-induced arthritis and in human fibroblast-like synoviocytes. Frontiers in Pharmacology. 2019;10 doi: 10.3389/fphar.2019.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S., Li M., Fang Y., Li Q., Liu J., Duan X., Liu Y., Wu R., Shi X., Wang Y., Jiang Z., Wang Y., Yu C., Wang Q., Tian X., Zhao Y., Zeng X. Chinese registry of rheumatoid arthritis (CREDIT): II. prevalence and risk factors of major comorbidities in Chinese patients with rheumatoid arthritis. Arthritis Research & Therapy. 2017;19(1) doi: 10.1186/s13075-017-1457-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalashnikova I., Chung S.J., Nafiujjaman M., Hill M.L., Siziba M.E., Contag C.H., Kim T. Ceria-based nanotheranostic agent for rheumatoid arthritis. Theranostics. 2020;10(26):11863–11880. doi: 10.7150/thno.49069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C., Lee J.H., Kim W., Li D., Kim Y., Lee K., Kim S.K. The suppressive effects of cinnamomi cortex and its phytocompound coumarin on oxaliplatin-induced neuropathic cold allodynia in rats. Molecules. 2016;21(9) doi: 10.3390/molecules21091253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.Y., Lim K., Kim K.H., Kim J.H., Choi J.S., Shim S.C. N-3 polyunsaturated fatty acids restore Th17 and treg balance in collagen antibody-induced arthritis. PLoS One. 2018;13(3) doi: 10.1371/journal.pone.0194331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klareskog L., Catrina A.I., Paget S. Rheumatoid arthritis. Lancet. 2009;373(9664):659–672. doi: 10.1016/S0140-6736(09)60008-8. [DOI] [PubMed] [Google Scholar]
- Lee Y.A., Choi H.M., Lee S.H., Hong S.J., Yang H.I., Yoo M.C., Kim K.S. Hypoxia differentially affects IL-1β-stimulated MMP-1 and MMP-13 expression of fibroblast-like synoviocytes in an HIF-1α-dependent manner. Rheumatology. 2012;51(3):443–450. doi: 10.1093/rheumatology/ker327. [DOI] [PubMed] [Google Scholar]
- Li B.T., Zhang F.Z., Xu T.S., Ding R., Li P. Increasing production of matrix metalloproteinases, tumor necrosis factor-α, vascular endothelial growth factor and prostaglandin E2 in rheumatoid arthritis synovial fibroblasts by different adiponectin isoforms in a concentration-dependent manner. Cellular and Molecular Biology. 2015;61(7):27–32. [PubMed] [Google Scholar]
- Li X., Fu X., Gao Y., Li H., Wang W., Shen Y. Expression of tissue inhibitor of metalloproteinases-1 and B-cell lymphoma-2 in the synovial membrane in patients with knee osteoarthritis. Experimental and Therapeutic Medicine. 2018;15(1):885–889. doi: 10.3892/etm.2017.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Cao H., Yao M., Lei X. Effect of acupuncture combined with shenqi yigan decoction on liver function and T cell subsets in patients with HBV-induced liver fibrosis. American Journal of Translational Research. 2021;13(4):3409–3417. [PMC free article] [PubMed] [Google Scholar]
- Lin W.N., Su H.L., Li H.M., Peng D.H., Hong F.H., Zeng Y.N., Wang Q.H. Therapeutic mechanism of Huangqi Guizhi Wuwu Tang on rheumatoid arthritis. Chinese Journal of Experimental Traditional Medical Formulae. 2022;28(9):9–15. [Google Scholar]
- Liu J.W., Li Y.Y., Wang Y.H. Research on mechanism of Huangqi Guizhi Wuwu Decoction on collagen-induced arthritis in rats. Shanxi Journal of Traditional Chinese Medicine. 2017;33(1):52–54. [Google Scholar]
- Liu J.W., Wang Y.H., Li Y.Y., Zhang Y.G., Zhao L., Zhang R.N., Sun L.W. Effect of Huangqi Guizhi Wuwu Decoction on JAK-STAT signaling pathway in CIA model rats with rheumatoid arthritis. Lishizhen Medicine and Materia Medica Research. 2019;30(4):811–814. [Google Scholar]
- Liu W., Fan Y., Tian C., Jin Y., Du S., Zeng P., Wang A. Deciphering the molecular targets and mechanisms of HGWD in the treatment of rheumatoid arthritis via network pharmacology and molecular docking. Evidence-based Complementary and Alternative Medicine: eCAM. 2020;2020 doi: 10.1155/2020/7151634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Shi L., Wan Q., Wu Y., Huang D., Ou J., Liu Q., Guan X., Yang Y., Zhang X., Gao J. Huangqi Guizhi Wuwu Decoction attenuates podocyte cytoskeletal protein damage in IgA nephropathy rats by regulating AT1R/Nephrin/c-abl pathway. Biomedicine & Pharmacotherapy. 2021;142(5) doi: 10.1016/j.biopha.2021.111907. [DOI] [PubMed] [Google Scholar]
- Ma J.B., Ma Y., Zhao Z.J. Clinical study of Huangqi Guizhi Wuwu Decoction combined with yupingfeng powder in the treatment of rheumatoid arthritis. Journal of New Chinese Medicine. 2016;48(7):115–116. [Google Scholar]
- McInnes I.B., Schett G. The pathogenesis of rheumatoid arthritis. The New England Journal of Medicine. 2011;365(23):2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- Pang B., Zhao T.Y., Zhao L.H., Wan F., Ye R., Zhou Q., Tian F., Tong X.L. Huangqi Guizhi Wuwu Decoction for treating diabetic peripheral neuropathy: A meta-analysis of 16 randomized controlled trials. Neural Regeneration Research. 2016;11(8):1347–1358. doi: 10.4103/1673-5374.189202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szekanecz Z., Haines G.K., Lin T.R., Harlow L.A., Goerdt S., Rayan G., Koch A.E. Differential distribution of intercellular adhesion molecules (ICAM-1, ICAM-2, and ICAM-3) and the MS-1 antigen in normal and diseased human synovia. Their possible pathogenetic and clinical significance in rheumatoid arthritis. Arthritis and Rheumatism. 1994;37(2):221–231. doi: 10.1002/art.1780370211. [DOI] [PubMed] [Google Scholar]
- Tang S.W., Li X.L., Ma L., Zheng Y.F., Li C.Y., Cheng X.L., Cao P., Peng G.P. Research on chemical composition of Jiawei Huangqi Guizhi Wuwu Decoction based on HPLC fingerprint and LC-Q-TOF/MS. Chinese Traditional and Herbal Drugs. 2023;54(3):711–721. [Google Scholar]
- Ten Klooster P.M., de Graaf N., Vonkeman H.E. Association between pain phenotype and disease activity in rheumatoid arthritis patients: A non-interventional, longitudinal cohort study. Arthritis Research & Therapy. 2019;21(1) doi: 10.1186/s13075-019-2042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Fan Y., Xin P., Zhao Y., Deng H., Jia B. The efficacy and safety of Huangqi Guizhi Wuwu Decoction for rheumatoid arthritis: A protocol for systematic review and meta-analysis. Medicine. 2020;99(36) doi: 10.1097/MD.0000000000022011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Zhou X., Zhao Y., Xiao J., Lu Y., Shi Q., Wang Y., Wang H., Liang Q. Polyphyllin I ameliorates collagen-induced arthritis by suppressing the inflammation response in macrophages through the NF-κB pathway. Frontiers in Immunology. 2018;9 doi: 10.3389/fimmu.2018.02091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Chen T., Yang C., Li Q., Ma M., Xu H., Shi Q., Wang Y., Wang Y., Liang Q. Huangqi Guizhi Wuwu Decoction improves arthritis and pathological damage of heart and lung in TNF-tg mice. Frontiers in Pharmacology. 2022;13 doi: 10.3389/fphar.2022.871481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y., Jiang N., Liu T., Liu C., Xiao W., Liang L., Li T., Yu Y. The comparison of extraction methods of ganjiang decoction based on fingerprint, quantitative analysis and pharmacodynamics. Chinese Medicine. 2020;15 doi: 10.1186/s13020-020-00355-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J., Li S., Xiao L., Ouyang G., Zheng L., Gu Y., Gao C., Han X. Zoledronic acid ameliorates the effects of secondary osteoporosis in rheumatoid arthritis patients. Journal of Orthopaedic Surgery and Research. 2019;14(1) doi: 10.1186/s13018-019-1492-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Bouta E.M., Wood R.W., Schwarz E.M., Wang Y., Xing L. Utilization of longitudinal ultrasound to quantify joint soft-tissue changes in a mouse model of posttraumatic osteoarthritis. Bone Research. 2017;5 doi: 10.1038/boneres.2017.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X.F., Lin B., Wang P. The effect of HuangQiGuizhi Decoction on IL-1β and TNF-α in blood serum of AA rats. Progress in Modern Biomedicine. 2007;7(8):1202–1204. [Google Scholar]
- Yabe R., Chung S.H., Murayama M.A., Kubo S., Shimizu K., Akahori Y., Maruhashi T., Seno A., Kaifu T., Saijo S., Iwakura Y. TARM1 contributes to development of arthritis by activating dendritic cells through recognition of collagens. Nature Communications. 2021;12(1) doi: 10.1038/s41467-020-20307-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q., Feng M. Effects of Huangqi Guizhi Wuwu Decoction on serum IL-20 in rats with collagen-induced arthritis. Chinese Remedies & Clinics. 2012;12(7):880–883. [Google Scholar]
- Yap H.Y., Tee S.Z., Wong M.M., Chow S.K., Peh S.C., Teow S.Y. Pathogenic role of immune cells in rheumatoid arthritis: Implications in clinical treatment and biomarker development. Cells. 2018;7(10) doi: 10.3390/cells7100161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai Q.M., Dong J.Y., Zhang X.S., He X.N., Fei D.D., Jin Y., Li B., Jin F. Mesenchymal stem cells enhance therapeutic effect and prevent adverse gastrointestinal reaction of methotrexate treatment in collagen-induced arthritis. Stem Cells International. 2021;2021 doi: 10.1155/2021/8850820. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.