Abstract
Background
Alpha‐synuclein (SNCA) copy number variations (CNV) have been certified as a causative mutation in patients with familial and sporadic Parkinson's disease (PD).
Case
We report three SNCA duplication cases diagnosed as PD. Through whole‐exome sequencing, we identified a de novo 4.56 Mb repeated region in one patient and a 2.50 Mb repeated region in familial PD with two patients.
Literature review
In review of previous cases, we suggest that aggressive behavior is more remarkable in CNV4 patients. Meanwhile, frequency of cognition decline and dementia were slightly increased in CNV4 patients. We also illustrate a younger onset age in offspring than parent in familial SNCA multiplication PD cases. No difference was observed in disease duration between parent and offspring generation.
Conclusions
Our findings demonstrated the clinical and genetic characteristics in PD with SNCA multiplication and provided strong evidence for genetic anticipation. These results may be instructive for future disease diagnosis and genetic counseling.
Keywords: SNCA, Parkinson's disease, copy number variation, genotype–phenotype
Alpha‐synuclein (SNCA), the dominant protein in lewy bodies, is typical of cytoplasmic inclusions found in Parkinson's disease (PD), Lewy body dementia, and other diseases called synucleinopathies. Missense mutation and copy number variants (CNV) of the α‐synuclein SNCA gene have been reported as a genetic cause of autosomal dominant Parkinson's disease. 1 , 2 , 3 Compared with missense mutations in SNCA, patients with SNCA CNV had more prominent problems in psychiatric signs and cognitive declination. 4 , 5 Previous result demonstrated the gene dosage of α‐synuclein in multiplication mutation patients, which is the higher expression level of α‐synuclein, the more malignant the PD phenotype. 6 However, a meta‐analysis of SNCA multiplications showed neither the multiplication size nor the number of genes included was associated with motor symptom onset or dementia in patients. 7 Clinical heterogeneity and low penetrance in SNCA multiplication patients also made it complicated to investigate and summarize. 8 , 9 So far, the genotype–phenotype correlation remains unclear. Here, we report three patients with Parkinson's disease caused by SNCA gene duplication, and describe the frequency of clinical manifestations in patients carrying SNCA multiplication.
Patients and Materials
Patients
Four hundred and thirty Parkinson's disease patients underwent genetic testing. PD was diagnosed by the United Kingdom Parkinson's Disease Society Brain Bank criteria. The criteria for genetic testing in Parkinson's disease include: (i) early‐onset PD patients; (ii) patients with family history; (iii) patients with excessive iron deposition in basal ganglia. PD patients had previously been identified carrying known PD‐causative genes were excluded. All patients were native Chinese. Two patients with PD were recruited from the outpatient clinic of the Department of Neurology, Qilu hospital of Shangdong University. Family members of both patients were enrolled. Clinical presentations including medical history, physical examination, and scale assessment as well as biological sample including peripheral blood were collected from all subjects. This study was approved by the Ethics Committee of Shandong University's Qilu Hospital, and written informed consent was obtained from all family members or legal representative.
Genetic Analysis
Blood samples of patients and family members were collected. DNA was isolated from peripheral blood using DNA Isolation Kit (Blood DNA Kit V2, CW2553). Whole‐exome sequencing was performed for the family members. Genome localization was analyzed according to GRCh37(hg19). Sample dilution, flow cell loading and sequencing were performed according to the Illumina specifications. DNA libraries were sequenced on the illumina novaseq platform as paired‐end 200‐bp reads. MLPA was carried out through standard protocol. In brief, DNA was denatured with heat and hybridized with probes. Ligation of hybridized probes, PCR amplification of ligated probes, fragment separation and further analysis were carried out.
Statistical Analysis
Paired T test was used for genetic anticipation analysis with the use of Graphpad prism 8.0. Comparison of Survival Curves was carried out with Log‐rank (Mantel‐Cox) test in Graphpad prism 8.0. For categorical variables including motor and non‐motor symptoms, we used Fishers exact test with the use of IBM SPSS Statistics 25. Differences with p < 0.05 were considered statistically significant.
Case Series
Case 1
Proband 1: The patient is a 37‐year‐old woman who developed rigidity in right upper and lower limbs, rest tremor in right hand, bradykinesia at the age of 36 (Fig. 1A). She also complained of decreased sense of smell. During the past year, she did not take any medication due to pregnancy and thus the above symptoms gradually aggravated. Tremor spread to her left hand and right leg. She also developed postural instability presenting as dragging right foot. She had no REM sleep behavior disturbance (RBD), constipation, autonomic dysfunction or psychological symptoms. Physical examination showed dystonia in right arm. Her UPDRS III score was 5 at the age of 37. Brain MRI was unremarkable. Her cognition ability was normal based on Mini–Mental Status Examination (MMSE). Recently, she was prescribed with 150 mg levodopa per day and her symptoms greatly relieved.
Figure 1.
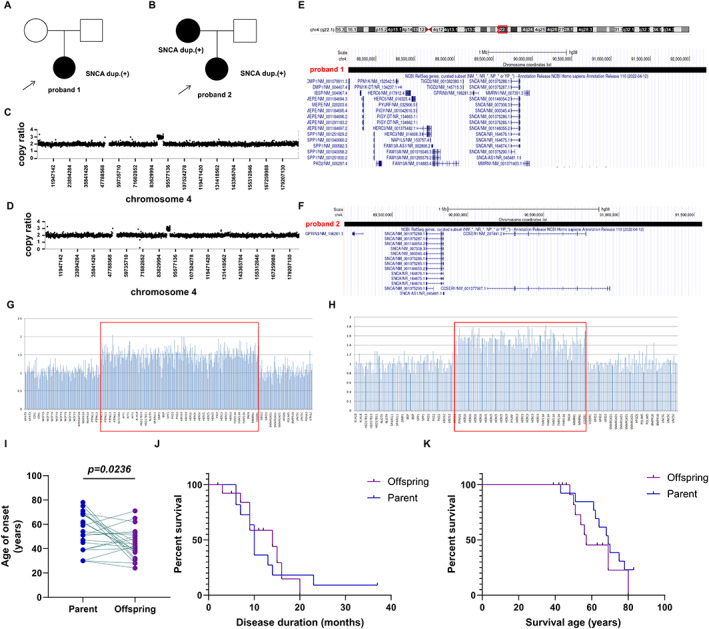
Genetic information of SNCA duplication patients and genotype–phenotype correlation of SNCA multiplication patients. Genogram of proband 1 (A) and proband 2 (B). Arrow indicates the proband. Black color indicates affected individuals. Whole‐exome sequencing identified multiplication in chromosome 4 in proband 1 (C) and proband 2 (D). (E) A genome review of SNCA duplication in proband 1. (F) A genome review of SNCA duplication in proband 2. MLPA results of proband 1 (G) and proband 2 (H). (I) Paired comparison of onset ages between parents and offspring revealed genetic anticipation. (J) Survival curve of disease duration in parents and offspring. (K) Survival curve of age of death in parents and offspring.
Whole‐exome sequencing revealed repeated mutations in chr4:87679397–92007182 (Fig. 1C). The duplicated region is 4.56 Mb in length and contains 50 genes including SNCA (Fig. 1E). It is further confirmed by MLPA (Fig. 1G). Genetic testing carried out on her parents showed negative results of SNCA duplication.
Case 2
Proband 2: The patient is a 51‐year‐old woman who presented with facial stiffness and rigidity in her left leg at the age of 46 and was diagnosed as PD (Fig. 1B). She had no tremor but presented with bradykinesia. She also had postural instability presenting as turning difficulty and leaning to the right. Hyposmia, RBD and constipation developed approximately at the age of 47. Orthostatic hypotension or psychological symptoms were not observed. Her UPDRS III score was 17 at the age of 51. Cognition ability was normal based on MMSE. After administration of levodopa (300 mg/day), her symptoms relieved, but suffered from “on–off” phenomenon. Patient 3, the mother of proband 2, is a 73‐year‐old woman who had tremor in right upper limb at the age of 68 and was diagnosed as PD. Tremor gradually affected bilateral upper and lower limbs. The patient had no RBD, constipation, hyposmia, or cognitive impairment. Levodopa did not improve the above symptoms apparently. In proband 2 and her mother, through whole‐exome sequencing, a 2.50 Mb duplication at 4q22.1 were detected while proband's father was normal (Fig. 1D). The 2.50 Mb repeated region contains a total of 23 genes including SNCA (Fig. 1F), and was further confirmed by MLPA (Fig. 1H).
Literature Review
In review of previous literatures, we summarized clinical characteristics of SNCA duplication and triplication cases reported in PD (Table. 1). SNCA multiplication carriers were classified into CNV3 (heterozygous for duplication) (n = 53) and CNV4 (heterozygous for triplication and homozygous for duplication) (n = 17). Genetic verified patients with complete record of motor and non‐motor symptoms were included. Bradykinesia, rigidity and resting tremor were the most common motor symptoms, with the frequency of 75.47%, 71.70%, 67.92% in CNV3 patients and 76.47%, 76.47% 64.71% in CNV4 patients. Gait disturbance was also commonly seen, accounting for 51.92% of CNV3 patients and 37.74% of CNV4 patients. Less than half of patients had postural instability. The non‐motor symptoms showed great variation. Aggressive behavior (3.77% in CNV3, 23.53% in CNV4) were more prominent in CNV4 patients. Cognitive decline and dementia were more frequent in CNV4 patients, however showed no significant differences between CNV3 patients. Motor symptoms and other non‐motor symptoms (depression, anxiety, jealousy, visual hallucination, delusion, orthostatic hypotension or tachycardia, RBD, hyposmia or anosmia and gastrointestinal dysfunction) showed no differences between mutation types.
TABLE 1.
Comparison of clinical features between SNCA CNV3 and CNV4 patients
| Clinical features | CNV3 | CNV4 | P value |
|---|---|---|---|
| Motor symptoms | |||
| Bradykinesia | 40/53 (75.47%) | 13/17 (76.47%) | n.s |
| Rigidity | 38/53 (71.70%) | 13/17 (76.47%) | n.s |
| Resting tremor | 36/53 (67.92%) | 11/17 (64.71%) | n.s |
| Postural instability | 21/53 (38.62%) | 3/17 (17.65%) | n.s |
| Gait disturbance | 27/53 (51.92%) | 6/17 (35.29%) | n.s |
| Non‐motor symptoms | |||
| Depression | 24/53 (45.28%) | 10/17 (58.82%) | n.s |
| Anxiety | 6/53 (11.32%) | 0/17 (0.00%) | n.s |
| Jealous | 1/53 (1.89%) | 2/17 (11.76%) | n.s |
| Aggressive behavior | 2/53 (3.77%) | 4/17 (23.53%) | P = 0.028 |
| Visual hallucination | 32/53 (60.38%) | 7/17 (41.18%) | n.s |
| Delusion | 5/53 (9.43%) | 2/17 (11.76%) | n.s |
| Orthostatic hypotension/tachycardia | 16/53 (30.19%) | 5/17 (29.41%) | n.s |
| Cognitive decline | 19/53 (35.85%) | 7/17 (41.18%) | n.s |
| Dementia | 10/53 (18.87%) | 6/17 (35.29%) | n.s |
| RBD | 17/53 (32.08%) | 5/17 (29.41%) | n.s |
| Hyposmia/anosmia | 13/53 (24.53%) | 4/17 (23.53%) | n.s |
| Gastrointestinal dysfunction | 12/53 (23.08%) | 4/17 (23.53%) | n.s |
Next, we analyzed the age at onset of PD with SNCA multiplication (both CNV3 and CNV4). There were 22 parent/child pairs for PD with SNCA multiplication included (Table S1). In the age at onset of PD, a statistically significant difference was found in 22 parent/child pairs (Fig. 1I). Information of disease duration and age of death (suicide was excluded) in 11 parents and 15 offspring (due to more than one offspring patient in family) were collected. However, no significant difference was observed in either disease duration (Fig. 1J) or age of death (Fig. 1K).
Discussion
We here demonstrated three Chinese patients carrying SNCA duplications diagnosed as PD. Genetic analysis of previously reported SNCA repeat patients, geographically distributed in Asia, Europe, and America, showed that the repeat genome ranged from 139 Kb to 41.2 Mb, containing 1–150 genes. 4 The majority of these patients showed a typical Parkinson's phenotype, with about half developing motor symptoms in the early course of the disease, and many patients also described non‐motor symptoms such as cognitive deficits, depression, hallucinations, RBD, and autonomic nervous dysfunction. 5 Previous research indicated that SNCA duplication resembles idiopathic PD without early development of dementia while triplication patients possess more severe symptoms. 10 In this report, we conclude that both duplication and triplication patients are characterized with parkinsonism, cognition impairment and psychiatric symptoms. Genetic research on Chinese population listed clinical features of SNCA carriers and indicated that compared to non‐carriers, SNCA duplication patients have more severe cognition decline, depression and autonomic dysfunction than heterozygous patients. 11 Trinh et al 12 compared clinical differences between patients with a triplication (n = 11), missense mutation (p.A53T; n = 53), and duplication (n = 47). They concluded that, compared to missense mutation carriers, psychotic symptoms and sleeping problems are more frequent in duplication/triplication carriers. Results also showed that cognitive decline frequency were relatively the same among duplication/triplication and missense mutation carriers. Here, we conclude that aggressive behaviors were more remarkable in CNV4 patients and that frequency of cognitive decline and dementia were slightly increased in CNV4 patients.
The three SNCA repeat patients mentioned in this paper showed typical Parkinson's symptoms, including tremor, bradykinesia, postural instability and gait disturbance. Limited non‐motor symptoms were observed in proband 1 and patient 2, with only hyposmia and/or constipation, RBD. There were no significant cognitive abnormalities in either patient. It can be seen that the clinical manifestations of repeated SNCA patients are variable. The repeated genomic regions of the two patients were concentrated in the vulnerable 4Q22 region, but the size of the range was significantly different. The length of the region and the number of genes involved in the repeat mutation in proband 2 were less than those in proband 1. However, proband 2 presented with more complex and severe non‐motor symptoms. Thus, there was no clear correlation between the size of the repeat region and the resulting clinical phenotype.
Anticipation of onset age is a characteristic of disorders with dynamic mutations and has also been reported in PD families with SNCA A53T mutation. 13 Here, in view of the anticipation in proband 2 compared to her mother, we carried out further analysis to reveal the phenotypic divergence between generations. Based on previous reported cases with known family history, 3 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 our result demonstrated that most younger generations have earlier onset age compared to their elder generation despite identical repeated regions throughout the pedigree. As shown in Table S1, SNCA multiplication were confirmed in most patients, however, genetic information for few patients were missing. Other pedigrees with ambiguous onset age, though not included in analysis, also showed anticipation. 9 Moreover, there is one family with two affected offspring at a relatively young age, but their transmitting parent, who carried the same mutation, were asymptomatic. 18 , 24 In fact, the low penetrance (lower than 50% in reported cases) 8 , 9 of SNCA multiplication may cause many asymptomatic carriers in parent generations to be undiagnosed. Thus, genetic anticipation might be more significant than currently analyzed. In contrast to onset age, disease course between generations showed no differences. Most patients died from respiratory infection due to being long‐term bedridden. We speculate that: (1) improvements in nutrition and healthcare contributed to a longer disease course and lifespan in offspring generations; (2) the onset age of parent generations is generally older, mostly in their 60s, which led to a shorter disease course even if they live an ordinary lifetime. However, such an analysis needs to be further confirmed by ruling out other factors that may affect the onset of PD, including environmental factors.
While our study demonstrated the phenotypes of SNCA CNV3 and CNV4 patients, there are several limitations. First, the limited sample size due to rare occurrence of SNCA multiplication may result in incomprehensive conclusions. Second, the presence of psychotic symptoms and cognitive decline/dementia could be impacted by confounding variables, such as age, disease duration and medications. Detailed clinical information of all patients and stepwise regression may help us better illustrate the phenotypic characteristics of SNCA multiplication‐related PD.
In conclusion, we report three Chinese SNCA duplication patients and described the phenotype associated with SNCA repeats. Moreover, we provided evidence supporting genetic anticipation in SNCA multiplication related PD pedigrees. Our findings provide fundamental guidance for further research on disease phenotypes.
Author Roles
(1) Research project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript: A. Writing of the first draft, B. Review and Critique.
R.N.D.: 1A, 1B, 2A, 2B, 3A
G.Y.L.: 1B, 3A
Y.M.L.: 1C, 2C, 3B
Y.L.H.: 2B, 3B
P.Z.L.: 1B, 3B
B.H.Z.: 2B, 3B
Disclosures
Ethical Compliance Statement: This study is approved by Ethics Committee of Shandong University's Qilu Hospital. Informed consent was obtained from the patients and their family members. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflicts of Interest: This work is supported by National Natural Science Foundation of China (No. 82101487, 82171245), Natural Science Foundation of Shandong Province, China (ZR2021QH161) and Taishan Scholar Program of Shandong Province (tsqn202211318). The authors declare that there are no conflicts of interest relevant to this work.
Financial Disclosures for the Previous 12 Months: The authors declare that there are no additional disclosures to report.
Supporting information
Table S1. Onset age and copy number variations of offspring and parent generation.
Ruo‐Nan Duan and Gui‐Yu Liu contributed equally to this work.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request. Other data are available within the article.
References
- 1. Polymeropoulos MH, Lavedan C, Leroy E, et al. Mutation in the alpha‐synuclein gene identified in families with Parkinson's disease. Science 1997;276:2045–2047. [DOI] [PubMed] [Google Scholar]
- 2. Singleton AB, Farrer M, Johnson J, et al. Alpha‐synuclein locus triplication causes Parkinson's disease. Science 2003;302:841. [DOI] [PubMed] [Google Scholar]
- 3. Chartier‐Harlin MC, Kachergus J, Roumier C, et al. Alpha‐synuclein locus duplication as a cause of familial Parkinson's disease. Lancet 2004;364:1167–1169. [DOI] [PubMed] [Google Scholar]
- 4. Guo Y, Sun Y, Song Z, et al. Genetic analysis and literature review of SNCA variants in Parkinson's disease. Front Aging Neurosci 2021;13:648151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Konno T, Ross OA, Puschmann A, Dickson DW, Wszolek ZK. Autosomal dominant Parkinson's disease caused by SNCA duplications. Parkinsonism Relat Disord 2016;22(Suppl 1):S1–S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eriksen JL, Przedborski S, Petrucelli L. Gene dosage and pathogenesis of Parkinson's disease. Trends Mol Med 2005;11:91–96. [DOI] [PubMed] [Google Scholar]
- 7. Book A, Guella I, Candido T, et al. A meta‐analysis of alpha‐synuclein multiplication in familial parkinsonism. Front Neurol 2018;9:1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nishioka K, Hayashi S, Farrer MJ, et al. Clinical heterogeneity of alpha‐synuclein gene duplication in Parkinson's disease. Ann Neurol 2006;59:298–309. [DOI] [PubMed] [Google Scholar]
- 9. Ahn TB, Kim SY, Kim JY, et al. Alpha‐synuclein gene duplication is present in sporadic Parkinson disease. Neurology 2008;70:43–49. [DOI] [PubMed] [Google Scholar]
- 10. La Cognata V, Morello G, D'Agata V, Cavallaro S. Copy number variability in Parkinson's disease: assembling the puzzle through a systems biology approach. Hum Genet 2017;136:13–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhao Y, Qin L, Pan H, et al. The role of genetics in Parkinson's disease: a large cohort study in Chinese mainland population. Brain 2020;143:2220–2234. [DOI] [PubMed] [Google Scholar]
- 12. Trinh J, Zeldenrust FMJ, Huang J, et al. Genotype‐phenotype relations for the Parkinson's disease genes SNCA, LRRK2, VPS35: MDSGene systematic review. Mov Disord 2018;33:1857–1870. [DOI] [PubMed] [Google Scholar]
- 13. Huang Y, Hayes M, Harding AJ, et al. Anticipation of onset age in familial Parkinson's disease without SCA gene mutations. Parkinsonism Relat Disord 2006;12:309–313. [DOI] [PubMed] [Google Scholar]
- 14. Elia AE, Petrucci S, Fasano A, et al. Alpha‐synuclein gene duplication: marked intrafamilial variability in two novel pedigrees. Mov Disord 2013;28:813–817. [DOI] [PubMed] [Google Scholar]
- 15. Ikeuchi T, Kakita A, Shiga A, et al. Patients homozygous and heterozygous for SNCA duplication in a family with parkinsonism and dementia. Arch Neurol 2008;65:514–519. [DOI] [PubMed] [Google Scholar]
- 16. Uchiyama T, Ikeuchi T, Ouchi Y, et al. Prominent psychiatric symptoms and glucose hypometabolism in a family with a SNCA duplication. Neurology 2008;71:1289–1291. [DOI] [PubMed] [Google Scholar]
- 17. Nishioka K, Ross OA, Ishii K, et al. Expanding the clinical phenotype of SNCA duplication carriers. Mov Disord 2009;24:1811–1819. [DOI] [PubMed] [Google Scholar]
- 18. Nan H, Takaki R, Maruyama T, Baba Y, Ohara S, Shindo K, Takiyama Y. Orthostatic hypotension as a core symptom in a Japanese family harboring SNCA duplication. Parkinsonism Relat Disord 2020;81:28–30. [DOI] [PubMed] [Google Scholar]
- 19. Zafar F, Valappil RA, Kim S, et al. Genetic fine‐mapping of the Iowan SNCA gene triplication in a patient with Parkinson's disease. NPJ Parkinsons Dis 2018;4:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Olgiati S, Thomas A, Quadri M, et al. Early‐onset parkinsonism caused by alpha‐synuclein gene triplication: clinical and genetic findings in a novel family. Parkinsonism Relat Disord 2015;21:981–986. [DOI] [PubMed] [Google Scholar]
- 21. Fuchs J, Nilsson C, Kachergus J, et al. Phenotypic variation in a large Swedish pedigree due to SNCA duplication and triplication. Neurology 2007;68:916–922. [DOI] [PubMed] [Google Scholar]
- 22. Du YJ, Shen Y, Wang YX, et al. Clinical variability in Chinese families with Parkinson disease and SNCA duplication, including the shortest 139kb duplication. Parkinsonism Relat Disord 2019;68:60–62. [DOI] [PubMed] [Google Scholar]
- 23. Wurster I, Quadalti C, Rossi M, et al. Linking the phenotype of SNCA triplication with PET‐MRI imaging pattern and alpha‐synuclein CSF seeding. NPJ Parkinsons Dis 2022;8:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kojovic M, Sheerin U‐M, Rubio‐Agusti I, et al. Young‐onset parkinsonism due to homozygous duplication of α‐synuclein in a consanguineous family. Mov Disord 2012;27:1829–1830. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Onset age and copy number variations of offspring and parent generation.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request. Other data are available within the article.


