Abstract
The present study investigated the antimicrobial and anti‐biofilm effects of indigenous Lactobacillus probiotic strains on Pseudomonas aeruginosa isolated from burn wound infection in laboratory conditions. The effect of 7 probiotic strains isolated from infant faeces on the pathogenicity factors of P. aeruginosa, including protease, elastase, antibiofilm and antipyocyanin was measured. Also, diffusion methods in the well and micro broth dilution were used to evaluate the antimicrobial activity of probiotics. All tests were performed in triplicate. A negative control and a positive control were used for each test. SPSS version 22 software was used for statistical analysis, and a p < 0.05 was considered statistically significant. A total of 30 clinical isolates of P. aeruginosa were isolated. The elastolytic activity of P. aeruginosa isolates decreased after adding Cell free supernatant (CFS) of each Lactobacillus. L1, L4, L5, and L6 strains had a 100% inhibitory effect on pathogen isolates. L3 and L7 strains had the lowest inhibitory effect. The inhibitory effect of CFS extracted from lactobacilli on protease production by P. aeruginosa. L1, L4, L5, and L6 strains had an inhibitory effect on all tested isolates. L2, L3, and L7 strains had a less inhibitory effect. L4 strain had the highest inhibitory effect on pyocyanin production by P. aeruginosa (50%), followed by L5 (43.3%), L1 (40%), and L6 (23.3%) strains. L3 and L7 strains had no inhibitory effect on the pyocyanin production of P. aeruginosa isolates. It was found that the CFS of 4 isolates (L1, L4, L5, and L6) was the most active extract and had a 100% inhibitory effect against biofilm formation of all P. aeruginosa strains. The L3 strain had the least inhibitory effect against the biofilm formation of pathogens. Overall, this study showed that probiotics could be promising alternatives to combat the pathogenicity of P. aeruginosa in burn wounds.
Keywords: burns, Lactobacillus, probiotics, Pseudomonas aeruginosa, wounds and injuries
1. INTRODUCTION
Burn is a health problem that happens all over the world and it has inappropriate effects on society. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 Burns can be defined as damage to the skin or any organic tissue that is mainly caused by fire, electricity, radioactive, radiation, and chemical substances. 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 Burn injuries produce some of the most painful patient experiences 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 and may result in unpleasant physical and psychological outcomes among patients. 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 Most burn‐related deaths are caused by burn wound infections, which are the most common complications in burn patients. 65 There are a variety of bacteria that can grow in a burn wound because of its protein‐rich and avascular environment. 65 , 66 Burn wound infections are commonly caused by Pseudomonas aeruginosa. 65 This bacterium is an aerobic gram‐negative opportunistic pathogen that generally infects immunocompromised patients. 66 , 67 There has been evidence that P. aeruginosa has been found on a variety of hospital surfaces, including mattresses and the hands of healthcare workers. 65 Pseudomonas species produce many pathogenic factors, including adhesion, lipopolysaccharide, flagella, pili, endotoxin, exotoxin A, exoenzyme S, hemolysins, iron‐binding proteins, type III secretion system, alginate, pyocyanin, proteases, and elastase. 65 , 68 , 69 These factors mediate microbial stimulation, adhesion, escape, and destruction of tissue cells. 65 Protease‐containing strains generally cause more virulent infections. P. aeruginosa secretes several proteases (protease IV, elastase B, elastase A, and alkaline protease) that can degrade a wide range of host proteins such as fibrinogen, elastin, collagen, and plasminogen. 70 P. aeruginosa has become naturally or acquired resistant to many antimicrobial agents. 68 The widespread use of antibiotics has increased the antibiotic resistance in bacteria worldwide. The emergence of multidrug‐resistant (MDR), XDR, and especially PDR is a serious problem in the treatment of hospitalized patients. 71 , 72 It is also known that some bacteria, such as P. aeruginosa, exist in burn wounds as biofilms. 65 A biofilm is a highly organized community of bacterial cells that attaches to foreign surfaces 65 and can cause chronic infections in humans. 73 , 74 The greater severity of Pseudomonas infections in humans is related to their ability to form biofilms. 73 Biofilms reduce the effectiveness of antibiotics and cause resistance to the responses of the host's natural immune system. 65 , 72 Because of limited therapeutic options, treating wounds infected with P. aeruginosa has become difficult. 68 Colistin (polymyxin E) is the last treatment option against MDR P. aeruginosa strains. The emergence of resistance to colistin has been reported in several countries, including Denmark, the United Kingdom, and Australia. 75 Therefore, further research is necessary to develop new antibiotics and to identify alternative treatment methods. It is being studied whether probiotics, phages, or herbal remedies can be used to treat this pathogen without using antibiotics. 75 Probiotics are live microbial species that, when administered in sufficient quantities, have positive effects on the host's body. 73 These microorganisms can change the flora of the environment where they exist. 68 In addition, probiotics can be effective in fighting and preventing burn wound infections. 65 Probiotic bacteria have anti‐inflammatory and regenerative activities in treating skin damage. 71 The therapeutic role of probiotics has been proven through the production of antimicrobial agents, pathogen elimination, and immune modulation. 70 These bacteria can produce a major group of antimicrobial compounds, including bacteriocins, which have a wide range of effects against pathogens. 66 Probiotics may be an effective alternative to help antibiotics when treating patients with Pseudomonas infections. 66 Lactic acid bacteria (LAB) such as Lactobacillus are well‐known examples of probiotics showing antiseptic properties, immune system improvement, tissue repair, and angiogenesis. 68 It has also been shown that some lactobacilli can have antimicrobial effects against P. aeruginosa. 67 Based on research, non‐antibiotic antimicrobial agents alone or in combination with antibiotics can play an important role in disease management. 75
2. RESEARCH QUESTIONS
The study aimed to answer the following research questions:
How is the antibacterial activity of cell free supernatant (CFS) against P. aeruginosa?
What are the effects of CFS against the elastase activity of P. aeruginosa?
What are the effects of lactic acid on protease activity of P. aeruginosa?
What are the effects of CFS on pyocyanin production of P. aeruginosa?
What are the effects of CFS on biofilm formation of P. aeruginosa?
2.1. Aim
Therefore, the present study investigated the antimicrobial and anti‐biofilm effects of indigenous Lactobacillus probiotic strains on P. aeruginosa isolated from burn wound infection in laboratory conditions.
3. METHODS
3.1. Collection and isolation of pathogens
This cross‐sectional study was conducted from May to September 2021 in a teaching and remedial hospital in the north of Iran. Ethical approval for this study (IR.GUMS.REC.1400.004) was provided by the ethics and research committee of Guilan University of Medical Sciences. P. aeruginosa bacteria were clinically isolated from hospitalized burn wound patients. First, the samples obtained from the burn wound were grown in Tryptic Soy Broth (TSB) overnight at 37°C under aerobic conditions. The isolated bacteria were identified based on colony morphology, gram staining, and standard biochemical tests, including oxidase test, catalase test, indole test, Methyl Red and Voges Proskauer (MR‐VP) test, motility test, citrate test and sugar fermentation (TSI) and pigment production and growth on cetrimide agar. All phenotypically confirmed isolates were identified by amplification of P. aeruginosa algD gene with specific primers For algD (5′‐TTCCCTCGCAGAGAAAACATC‐3′) and Rev‐algD (5′‐CCTGGTTGATCAGGTCGATCT‐3′). 76
3.2. Preparation of CFS from lactic acid bacteria
In this study, seven Lactobacillus strains derived from infant faeces, confirmed as probiotic strains in our previous study (IR.GUMS.REC.1398.016), were used. These Lactobacillus isolates were resistant to low pH and bile salt stress. First, the strains were taken out of the freezer as frozen stock at −70°C and incubated in 5 mL broth at 5°C for 24 h. Then, the strains were incubated under anaerobic conditions in De Man, Rogosa and Sharpe (MRS) broth at 37°C overnight. The CFS was collected by centrifugation (at 1000 rpm for 30 min) and sterilized by filtration through a 0.22 μm pore size filter. 77
3.3. Antimicrobial effect of probiotics against P. aeruginosa isolates
3.3.1. Agar well diffusion test
The antimicrobial activity of all seven probiotic strains was tested separately against 30 P. aeruginosa isolates. First, a suspension of 0.5 McFarland concentration of P. aeruginosa was cultured in Mueller Hinton agar medium in a spreadsheet. Wells with a diameter of 6 mm were drilled in the cultivated plates. Then 50 μL of CFS of Lactobacillus isolates were inoculated into the wells. After incubation of the plates for 24 h at 37°C, the diameter of the inhibition zone was measured. 78
3.3.2. Broth microdilution test
Broth microdilution assay was using to determine the minimum inhibitory percentage (MIP). A 96‐well microdilution plate was used. First, the CFSs were diluted with MRS broth and used at different percentages (i.e., 10, 20, 30, 40, 50%). Then, 100 μL of CFS was added to well and two‐fold serial dilutions were continued from the 2nd well to the 12th well. Finally, 100 μL of diluted P. aeruginosa suspension (0.5 McFarland) was added to all wells. After 24 h of incubation at 37°C, bacterial growth was evaluated. The positive control was the well without CFS, and the negative control was the well without bacteria. The MIP was considered the lowest CFS concentration that completely inhibited the growth of P. aeruginosa. 78
3.3.3. Elastase assay
Bacterial suspension of each P. aeruginosa strain (0.5 McFarland) was prepared in LB broth medium, and CFS was added to it. After overnight incubation, the supernatant was collected by centrifugation of the suspension at 5000 rpm for 15 min and sterile filtration (0.22 μm). The inhibitory effect of CFS on elastolytic activity was determined by adding Elastin Congo Red (ECR, 10 mg) to the supernatant. After 3 h of incubation and then centrifugation to remove insoluble elastin–Congo Red, the absorbance of the supernatant was measured at 450 nm using a spectrophotometer. 79
3.3.4. Protease assay
The combination of LB agar and skim milk was used for each strain to evaluate the protease activity of the probiotics. These media contained CFS. The media without CFS were considered as a negative control. A colony of the pathogen strain was cultured on the centre of the medium (agar spot method) and incubated overnight at 37°C. Proteolysis was assessed as the clearance zone around the colonies. 79
3.3.5. Pyocyanin assay
Overnight culture of P. aeruginosa strains (0.5 McFarland) was prepared in LB broth medium, and CFS was added. After 18 h of incubation, the supernatant was collected by centrifuging the suspension at 9000 rpm for 10 min and filtration (0.22 μm). The supernatant (5 mL) was mixed with 3 mL chloroform and mix with vortex for 10 s. The transparent chloroform layer was moved to a sterile tube, mixed with 1 mL of 0.2 M hydrochloric acid, and vortexed again for 10 s. After centrifugation (2000 rpm, 5 min), three layers were seen in each tube. The upper layer was transferred to another tube, and pyocyanin concentration was measured at a wavelength of 520 nm. 79
3.4. Measuring biofilm formation
Pseudomonas aeruginosa strains were incubated overnight in tryptic soy broth (TSB) containing 0.25% glucose. Then this fresh culture was transferred to 96‐well polystyrene microtiter plates at a concentration of 0.5 McFarland (1:100). Hundred microliters of diluted probiotic CFS was added to the wells and incubated at 37°C for 24 h. Plankton bacteria from each well were washed 3 times with sterile distilled water and dried. Then the wells were stained with 125 μL of 0.1% crystal violet solution for 15 min. After washing three times and drying, 125 μL of 30% glacial acetic acid were added to the wells. Incubation was done for 15 min at room temperature, and absorbance was measured at 570 nm. 79
3.5. Statistics
All tests were performed in triplicate. A negative control and a positive control were used for each test. SPSS version 22 software was used for statistical analysis, and a p value <0.05 was considered statistically significant.
4. RESULTS
In this study, 30 P. aeruginosa strains were isolated, mainly collected from male patients (76.7%) in the age range of 31–50 years and with a Total Burn Surface Area (TBSA) of 21%–40%. In addition, the antimicrobial effects of seven probiotic strains (L1, L2, L3, L4, L5, L7, L7) on pathogen isolates were evaluated.
4.1. Antibacterial activity of CFS against P. aeruginosa
It was observed that the CFS of all probiotic strains had antipseudomonal activity. Probiotic strain L1 showed the highest inhibitory effect against P. aeruginosa. This strain has produced an average of 9.5 mm of inhibition against pathogen isolates in the well‐diffusion method. The lowest inhibition area was related to strain L7 (2.8 mm). In addition, the results of the MIP test showed that the highest inhibitory effect was related to lactobacilli at a concentration of 20% (Table 1).
TABLE 1.
MIP50/MBP90 of seven Lactobacillus CFS (%) against Pseudomonas aeruginosa.
| Lactobacillus strain supernatants | MIP50 (%) | MIP90 (%) | MIP range (%) |
|---|---|---|---|
| L1 | 20 | 20 | 20–40 |
| L2 | 40 | 20 | 20–60 |
| L3 | 60 | 40 | 20–60 |
| L4 | 20 | 20 | 10–40 |
| L5 | 20 | 20 | 20–60 |
| L6 | 20 | 20 | 10–60 |
| L7 | 40 | 20 | 20–80 |
4.2. Effects of CFS against the elastase activity of P. aeruginosa
The elastolytic activity of P. aeruginosa isolates decreased after adding CFS of each Lactobacillus. L1, L4, L5, and L6 strains had a 100% inhibitory effect on pathogen isolates. L3 and L7 strains had the lowest inhibitory effect (Figure 1).
FIGURE 1.
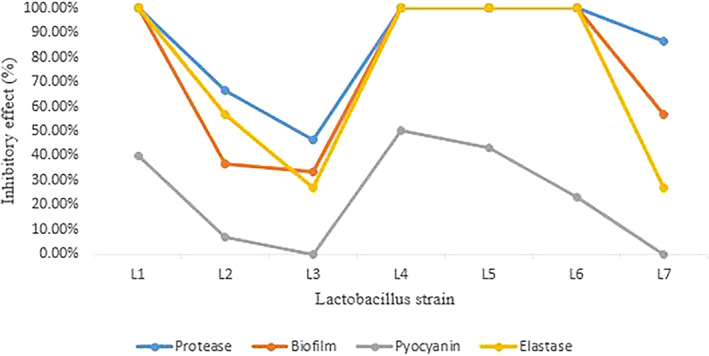
Effect of Lactobacillus CFS on Pseudomonas aeruginosa virulence factors.
4.3. The effects of lactic acid on protease activity of P. aeruginosa
Figures 1 and 2 show the inhibitory effect of CFS extracted from lactobacilli on protease production by P. aeruginosa. L1, L4, L5, and L6 strains had an inhibitory effect on all tested isolates. L2, L3, and L7 strains had a less inhibitory effect.
FIGURE 2.
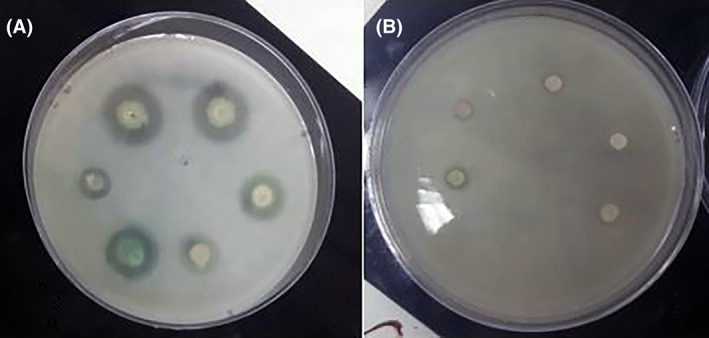
Antiprotease activity of P. aeuroginosa on LB agar with 2% skimmed milk. (A) Antiprotease activity of P. aeuroginosa before addition of CFS. (B) Antiprotease activity of P. aeuroginosa after addition of CFS.
4.4. Effects of CFS on pyocyanin production of P. aeruginosa
L4 strain had the highest inhibitory effect on pyocyanin production by P. aeruginosa (50%), followed by L5 (43.3%), L1 (40%), and L6 (23.3%) strains. L3 and L7 strains had no inhibitory effect on the pyocyanin production of P. aeruginosa isolates (Figure 1).
4.5. Effects of CFS on biofilm formation of P. aeruginosa
It was found that the CFS of 4 isolates (L1, L4, L5, and L6) was the most active extract and had a 100% inhibitory effect against biofilm formation of all P. aeruginosa strains. The L3 strain had the least inhibitory effect against the biofilm formation of pathogens (Figure 1).
5. DISCUSSION
As a result of the continuous use of classical methods to treat microbial infections, resistant strains have emerged and diseases have become more difficult to treat. Currently, research is focused on developing new antibiotics and alternative treatment methods without antibiotics. 79 In addition, probiotics have been considered a non‐toxic and available alternative to antibiotics. 72 It is important to note that probiotic effects are strain‐specific, so they are not equally effective, nor are they all recommended for the same health benefits. 69 This study investigated the in vitro effect of native Lactobacillus probiotics isolated from infant faeces on some important virulence factors of P. aeruginosa. These major virulence factors cause a wide range of pathogenic effects and extensive tissue damage. 80 Previous studies have shown that probiotics have properties that inhibit growth and virulence factors in P. aeruginosa. 81 Studies in Iran and Italy have reported that probiotic culture and their supernatant can inhibit P. aeruginosa burn infection. 71 , 82 , 83 The activity of 7 lactobacilli (L1, L2, L3, L4, L5, L6, L7) isolated in our study was wide, and most P. aeruginosa strains were inhibited. Based on the results, among the seven Lactobacillus isolates investigated, isolates No. 1 and 7 showed the highest (average 9.5 mm) and lowest (average 2.8 mm) inhibitory effect against P. aeruginosa isolates, respectively. In other studies, the growth inhibition zone diameter induced by probiotic lactobacilli supernatant was 17–20.3 mm. 66 , 84 The highest MIP value, that is, at 40% dilution of the supernatant, was observed for isolate No. 2. In the case of other isolates, the MIP value was noted at 20% dilution. In two studies conducted in Iran and Iraq, the reported MIC values for inhibiting P. aeruginosa isolates by probiotic strains were 31.25 and 50 μg/mL, respectively. 85 , 86 Despite the diversity of Lactobacillus strains used, the inhibitory activity of probiotic Lactobacillus isolates on pathogens was confirmed in these studies. It was found that the supernatant of Lactobacillus strains has an inhibitory effect on protease, elastase, and biofilm production of P. aeruginosa isolates so that strains No. 1, 4, 5, and 6 inhibit the protease, elastase, and biofilm of all pathogen isolates. Three other probiotic bacteria showed a less inhibitory effect. In line with our study, Kiymaci et al. in Turkey showed that a probiotic isolate (Pediococcus acidilactici) has anti‐elastase (46.89%) and anti‐protease (53.7%) activity against P. aeruginosa isolates. 79 A synergistic action of elastase and protease enzymes can cause destruction of a variety of immune system components, tissue invasion, and lung alveoli destruction. Therefore, inhibiting the activity of these enzymes can play a significant role in controlling the pathogenicity of P. aeruginosa. 67 , 79
Other studies have also shown the antimicrobial effect of probiotic lactobacilli such as L. plantarum (Valdez et al., 2005), L. fermentum (Varma et al., 2011), and L. fermentum and L. zeae (Alexandre et al., 2014), and L. plantarum (Eldeen et al., 2021) against the elastolytic activity of P. aeruginosa. 67 , 68 On the other hand, Alexandre in France reported that out of more than 80 Lactobacillus isolates tested, only five strains were able to significantly reduce the elastolytic activity of the control strain P. aeruginosa (PAO1). 87 We showed that the Lactobacillus supernatant significantly reduced biofilm and pyocyanin production in P. aeruginosa isolates. According to the findings, Lactobacillus strain No. 4 had the highest anti‐pyocyanin effect (50%), followed by strains 5, 1, 6, and 2, which showed an inhibitory effect of 6.7%–43.3%. Strains 3 and 7 had no inhibitory activity regarding pyocyanin production. Our results are consistent with two Argentinian studies that reported that Lactobacillus casei, acidophilus, and plantarum isolates inhibited biofilm production and pyocyanin concentration. 81 , 88 In a Turkish study, the probiotic strain P. acidilactici M7 also reported a significant antibiofilm (37%) and antipyocyanin (20%) effect against P. aeruginosa after addition. 79 Piocyanin is a pigment with inhibitory properties against many bacterial strains 79 and modifies the host's immune response. 81 A 50% or greater decrease in pyocyanin production is considered an extremely significant finding in P. aeruginosa infections. The pathogenesis of false infections and the toxic effects on biological systems in the infection area have been shown to be associated with this virulence factor. 79 Despite the differences in probiotics and methods between our study and those mentioned, there are some similarities. 67 The difference in the results can be explained by the different Lactobacillus strains used and their isolation source. 68 Lactobacillus supernatant was shown to be a promising alternative to combat P. aeruginosa pathogenicity in the present study.
5.1. Recommendations for future research
According to the findings of the present study, researchers in the future can consider the following recommendations for their research:
Identification of the genetic variations between different strains of Lactobacillus that exhibit efficacy against P. aeruginosa
The genetic engineering of Lactobacillus enhances its effectiveness in combating P. aeruginosa.
In vivo research to examine the impact of various strains of Lactobacillus on P. aeruginosa.
The identification of strains of P. aeruginosa that are resistant to Lactobacillus
Using artificial intelligence for accurate diagnosis of wound infections
5.2. Implications for clinical practice
Develop bio‐based drugs for treating burn wounds.
The development of dietary interventions to promote the healing of burn wounds.
The identification and production of antibiotics to aid in the treatment of burn wounds.
Development of complementary therapies for burn recovery.
6. CONCLUSION
Using probiotic bacteria in therapeutic approaches can be very effective in preventing or minimizing with hospital‐acquired infections. This study showed that the supernatant of isolated lactobacilli has a significant inhibitory effect on the activity of the main pathogenic factors of P. aeruginosa strains. Therefore, using the products of these probiotic strains (especially strains No. 1, 4, 5 and 6) as a safe and economical alternative to chemical drugs helps to better control P. aeruginosa infection in burn patients. The results of this research should be supported by future studies that these probiotics demonstrating that probiotic can be used as potent drugs in eradicating P. aeruginosa infections alone or in combination with anti‐pseudomonas treatments.
FUNDING INFORMATION
This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
CONFLICT OF INTEREST STATEMENT
The authors declare that there is no conflict of interest.
ETHICS STATEMENT
Ethical approval for this study (IR.GUMS.REC.1400.004) was provided by the ethics and research committee of Guilan University of Medical Sciences.
ACKNOWLEDGEMENTS
Not applicable.
Asadzadegan R, Haratian N, Sadeghi M, et al. Antibiofilm and antimicrobial activity of Lactobacillus cell free supernatant against Pseudomonas aeruginosa isolated from burn wounds. Int Wound J. 2023;20(10):4112‐4121. doi: 10.1111/iwj.14305
Contributor Information
Hadi Sedigh Ebrahim Saraei, Email: seddigh.hadi@gmail.com.
Meysam Hasannejad‐Bibalan, Email: meysam.hasannejad@gmail.com.
DATA AVAILABILITY STATEMENT
The datasets used during the current study are available from the corresponding author upon request.
REFERENCES
- 1. Mehrabi A, Falakdami A, Mollaei A, et al. A systematic review of self‐esteem and related factors among burns patients. Ann Med Surg. 2022;84:104811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mobayen M, Pour‐Abbas SE, Naghipour M, Akhoundi M, Ashoobi MT. Evaluating the knowledge and attitudes of the members of the medical community mobilization on first aid for burn injuries in Guilan, Iran. J Mazandaran Univ Med Sci. 2020;30(186):148‐155. [Google Scholar]
- 3. Mobayen M, Farzan R, Dadashi A, Rimaz S, Aghebati R. Effect of early grafting on improvement of lethal area index (la50) in burn patients: a 7‐year investigation in a burn referral Centre in the north of Iran. Ann Burns Fire Disasters. 2017;30(3):189‐192. [PMC free article] [PubMed] [Google Scholar]
- 4. Vaghardoost R, Ghavami Y, Sobouti B, Mobayen MR. Mortality and morbidity of fireworks‐related burns on the annual last Wednesday of the year festival (Charshanbeh Soori) in Iran: an 11‐year study. Trauma Mon. 2013;18(2):81‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Feizkhah A, Mobayen M, Habibiroudkenar P, et al. The importance of considering biomechanical properties in skin graft: are we missing something? Burns. 2022;48(7):1768‐1769. [DOI] [PubMed] [Google Scholar]
- 6. Hosseini SJ, Firooz M, Norouzkhani N, et al. Can the age group be a predictor of the effect of virtual reality on the pain management of burn patients? Burns. 2022;49:730‐732. [DOI] [PubMed] [Google Scholar]
- 7. Miri S, Hosseini SJ, Takasi P, et al. Effects of breathing exercise techniques on the pain and anxiety of burn patients: a systematic review and meta‐analysis. Int Wound J. 2023;20(6):2360‐2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Farzan R, Moeinian M, Abdollahi A, et al. Effects of amniotic membrane extract and deferoxamine on angiogenesis in wound healing: an in vivo model. J Wound Care. 2018;27(Sup6):S26‐S32. [DOI] [PubMed] [Google Scholar]
- 9. Haddadi S, Parvizi A, Niknama R, Nemati S, Farzan R, Kazemnejad E. Baseline characteristics and outcomes of patients with head and neck burn injuries; a cross‐sectional study of 2181 cases. Arch Acad Emerg Med. 2021;9(1):e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kazemzadeh J, Vaghardoost R, Dahmardehei M, et al. Retrospective epidemiological study of burn injuries in 1717 pediatric patients: 10 years analysis of hospital data in Iran. Iran J Public Health. 2018;47(4):584‐590. [PMC free article] [PubMed] [Google Scholar]
- 11. Tolouie M, Farzan R. A six‐year study on epidemiology of electrical burns in northern Iran: is it time to pay attention? World J Plast Surg. 2019;8(3):365‐371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vaghardoost R, Kazemzadeh J, Dahmardehei M, et al. Epidemiology of acid‐burns in a major referral hospital in Tehran, Iran. World J Plast Surg. 2017;6(2):170‐175. [PMC free article] [PubMed] [Google Scholar]
- 13. Parvizi A, Haddadi S, Ghorbani Vajargah P, et al. A systematic review of life satisfaction and related factors among burns patients. Int Wound J. 2023. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Doustahadi A, Beigee AM, Zare‐Kaseb A, Ghazanfari MJ. Suicidality after burn injuries: a significant overlooked challenge in burns survivors. J Nurs Rep Clin. 2023. In press. [Google Scholar]
- 15. Gari AA, Al‐Ghamdi YA, Qutbudden HS, Alandonisi MM, Mandili FA, Sultan A. Pediatric burns in Western Saudi Arabia. Saudi Med J. 2012;33(10):1106‐1110. [PubMed] [Google Scholar]
- 16. Sharma Y, Garg AK. Analysis of death in burn cases with special reference to age, sex and complications. J Punj Acad Forensic Med Toxicol. 2019;19(2):73. [Google Scholar]
- 17. Farzan R, Parvizi A, Haddadi S, et al. Effects of non‐pharmacological interventions on pain intensity of children with burns: a systematic review and meta‐analysis. Int Wound J. 2023. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Farzan R, Parvizi A, Takasi P, et al. Caregivers' knowledge with burned children and related factors towards burn first aid: a systematic review. Int Wound J. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Toolaroud PB, Nabovati E, Mobayen M, et al. Design and usability evaluation of a mobile‐based‐self‐management application for caregivers of children with severe burns. Int Wound J. 2023;n/a(n/a). In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Eftekhari H, Sadeghi M, Mobayen M, et al. Epidemiology of chemical burns: an 11‐year retrospective study of 126 patients at a referral burn centre in the north of Iran. Int Wound J. n/a(n/a). In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rangraz Jeddi F, Nabovati E, Mobayen M, et al. A smartphone application for caregivers of children with severe burns: a survey to identify minimum data set and requirements. J Burn Care Res. 2023;irad027. In press. [DOI] [PubMed] [Google Scholar]
- 22. Farzan R, Ghorbani Vajargah P, Mollaei A, et al. A systematic review of social support and related factors among burns patients. Int Wound J. 2023;n/a(n/a). In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Farzan R, Hosseini SJ, Firooz M, et al. Perceived stigmatisation and reliability of questionnaire in the survivors with burns wound: a systematic review and meta‐analysis. Int Wound J. 2023;n/a(n/a). In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Alizadeh Otaghvar H, Parvizi A, Ghorbani Vajargah P, et al. A systematic review of medical science students' knowledge and related factors towards burns first aids. Int Wound J 2023;n/a(n/a). In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yarali M, Parvizi A, Ghorbani Vajargah P, et al. A systematic review of health care workers' knowledge and related factors towards burn first aid. Int Wound J. 2023. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Farzan R, Hossein‐Nezhadi M, Toloei M, Rimaz S, Ezani F, Jafaryparvar Z. Investigation of anxiety and depression predictors in burn patients hospitalized at Velayat hospital, a newly established burn center. J Burn Care Res. 2022;44:irac184. [DOI] [PubMed] [Google Scholar]
- 27. Mobayen M, Torabi H, Bagheri Toolaroud P, et al. Acute burns during the COVID‐19 pandemic: a one‐year retrospective study of 611 patients at a referral burn Centre in northern Iran. Int Wound J. 2023. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rahbar Taramsari M, Mobayen M, Esmailzadeh M, et al. The effect of drug abuse on clinical outcomes of adult burn patients admitted to a burn center in the north of Iran: a retrospective cross‐sectional study. Bull Emerg Dent Traumatol. 2023;11(2):90‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zavarmousavi M, Eslamdoust‐Siahestalkhi F, Feizkhah A, et al. Gamification‐based virtual reality and post‐burn rehabilitation: how promising is that? Bull Emerg Dent Traumatol. 2023;11(2):106‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hamza Hermis A, Tehrany PM, Hosseini SJ, et al. Prevalence of non‐accidental burns and related factors in children: a systematic review and meta‐analysis. Int Wound J. 2023;n/a(n/a). In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Miri S, Mobayen M, Aboutaleb E, Ezzati K, Feizkhah A, Karkhah S. Exercise as a rehabilitation intervention for severe burn survivors: benefits & barriers. Burns. 2022;48:1269‐1270. [DOI] [PubMed] [Google Scholar]
- 32. Akhoondian M, Zabihi MR, Yavari S, et al. Radiation burns and fertility: a negative correlation. Burns. 2022;S0305‐4179(22):00223. [DOI] [PubMed] [Google Scholar]
- 33. Ghazanfari M, Mazloum S, Rahimzadeh N, et al. Burns and pregnancy during the COVID‐19 pandemic. Burns. 2022;48:2015‐2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Feizkhah A, Mobayen M, Ghazanfari MJ, et al. Machine learning for burned wound management. Burns. 2022;48:1261‐1262. [DOI] [PubMed] [Google Scholar]
- 35. Mobayen M, Feizkhah A, Ghazanfari MJ, et al. Sexual satisfaction among women with severe burns. Burns. 2022;48:1518‐1519. [DOI] [PubMed] [Google Scholar]
- 36. Mobayen M, Ghazanfari MJ, Feizkhah A, et al. Parental adjustment after pediatric burn injury. Burns. 2022;48:1520‐1521. [DOI] [PubMed] [Google Scholar]
- 37. Bazzi A, Ghazanfari MJ, Norouzi M, et al. Adherence to referral criteria for burn patients; a systematic review. Arch Acad Emerg Med. 2022;10(1):e43‐e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Miri S, Mobayen M, Mazloum SMH, et al. The role of a structured rehabilitative exercise program as a safe and effective strategy for restoring the physiological function of burn survivors. Burns. 2022;48:1521‐1523. [DOI] [PubMed] [Google Scholar]
- 39. Mobayen M, Ghazanfari MJ, Feizkhah A, Zeydi AE, Karkhah S. Machine learning for burns clinical care: opportunities & challenges. Burns. 2022;48(3):734‐735. [DOI] [PubMed] [Google Scholar]
- 40. Mobayen M, Feizkhah A, Ghazanfari MJ, et al. Intraoperative three‐dimensional bioprinting: a transformative technology for burn wound reconstruction. Burns. 2022;48(4):1023‐1024. [DOI] [PubMed] [Google Scholar]
- 41. Akhoondian M, Zabihi MR, Yavari S, et al. Identification of TGF‐β1 expression pathway in the improvement of burn wound healing. Burns. 2022;48(8):2007‐2010. [DOI] [PubMed] [Google Scholar]
- 42. Akhoondian M, Zabihi MR, Yavari S, et al. Burns may be a risk factor for endometriosis. Burns. 2022;49:476‐480. [DOI] [PubMed] [Google Scholar]
- 43. Asadi K, Aris A, Fouladpour A, Ghazanfari MJ, Karkhah S, Salari A. Is the assessment of sympathetic skin response valuable for bone damage management of severe electrical burns? Burns. 2022;48(8):2013‐2014. [DOI] [PubMed] [Google Scholar]
- 44. Salari A, Fouladpour A, Aris A, Ghazanfari MJ, Karkhah S, Asadi K. Osteoporosis in electrical burn injuries. Burns. 2022;48(7):1769‐1770. [DOI] [PubMed] [Google Scholar]
- 45. Takasi P, Falakdami A, Vajargah PG, et al. Dissatisfaction or slight satisfaction with life in burn patients: a rising cause for concern of the world's burn community. Burns. 2022;48:2000‐2002. [DOI] [PubMed] [Google Scholar]
- 46. Zabihi MR, Akhoondian M, Tajik MH, Mastalizadeh A, Mobayen M, Karkhah S. Burns as a risk factor for glioblastoma. Burns. 2022;49:236‐241. [DOI] [PubMed] [Google Scholar]
- 47. Mobayen M, Feizkhah A, Mirmasoudi SS, et al. Nature efficient approach; application of biomimetic nanocomposites in burn injuries. Burns. 2022;48(6):1525‐1526. [DOI] [PubMed] [Google Scholar]
- 48. Jeddi FR, Mobayen M, Feizkhah A, Farrahi R, Heydari S, Toolaroud PB. Cost analysis of the treatment of severe burn injuries in a tertiary burn center in Northern Iran. Iran Red Crescent Med J. 2022;24(5):e1522. [Google Scholar]
- 49. Mobayen M, Sadeghi M. Prevalence and related factors of electrical burns in patients referred to Iranian medical centers: a systematic review and meta‐analysis. World J Plast Surg. 2022;11(1):3‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mobayen M, Zarei R, Masoumi S, et al. Epidemiology of childhood burn: a 5‐year retrospective study in the referral burn center of Northern Iran Northern Iran. Caspian J Health Res. 2021;6(3):101‐108. [Google Scholar]
- 51. Haghdoost Z, Mobayen M, Omidi S. Predicting hope to be alive using spiritual experiences in burn patients. Ann Rom Soc Cell Biol. 2021;25(4):18957‐18962. [Google Scholar]
- 52. Mobayen M, Rimaz S, Malekshahi A. Evaluation of clinical and laboratory causes of burns in pre‐school children. J Curr Biomed Rep. 2021;2(1):27‐31. [Google Scholar]
- 53. Chukamei ZG, Mobayen M, Toolaroud PB, Ghalandari M, Delavari S. The length of stay and cost of burn patients and the affecting factors. Int J Burns Trauma. 2021;11(5):397. [PMC free article] [PubMed] [Google Scholar]
- 54. Khodayary R, Nikokar I, Mobayen MR, et al. High incidence of type III secretion system associated virulence factors (exoenzymes) in Pseudomonas aeruginosa isolated from Iranian burn patients. BMC Res Notes. 2019;12(1):1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rimaz S, Moghadam AD, Mobayen M, et al. Changes in serum phosphorus level in patients with severe burns: a prospective study. Burns. 2019;45(8):1864‐1870. [DOI] [PubMed] [Google Scholar]
- 56. Ghavami Y, Mobayen MR, Vaghardoost R. Electrical burn injury: a five‐year survey of 682 patients. Trauma Mon. 2014;19(4):e18748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Amir Alavi S, Mobayen MR, Tolouei M, et al. Epidemiology and outcome of burn injuries in burn patients in Guilan province, Iran. Qom Univ Med Sci J. 2013;7(5):35‐41. [Google Scholar]
- 58. Alavi CE, Salehi SH, Tolouei M, Paydary K, Samidoust P, Mobayen M. Epidemiology of burn injuries at a newly established burn care center in Rasht. Trauma Mon. 2012;17(3):341‐346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Norouzkhani N, Arani RC, Mehrabi H, et al. Effect of virtual reality‐based interventions on pain during wound care in burn patients; a systematic review and meta‐analysis. Arch Acad Emerg Med. 2022;10(1):e84‐e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Norouzkhani N, Ghazanfari MJ, Falakdami A, et al. Implementation of telemedicine for burns management: challenges & opportunities. Burns. 2022;49:482‐484. [DOI] [PubMed] [Google Scholar]
- 61. Farzan R, Firooz M, Ghorbani Vajargah P, et al. Effects of aromatherapy with Rosa damascene and lavender on pain and anxiety of burn patients: a systematic review and meta‐analysis. Int Wound J. 2023;20(6):2459‐2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Miri S, Hosseini SJ, Ghorbani Vajargah P, et al. Effects of massage therapy on pain and anxiety intensity in patients with burns: a systematic review and meta‐analysis. Int Wound J. 2023;20(6):2440‐2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Parvizi A, Haddadi S, Atrkar Roshan Z, Kafash P. Haemoglobin changes before and after packed red blood cells transfusion in burn patients: a retrospective cross‐sectional study. Int Wound J. 2023;20(6):2269‐2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mobayen M, Ghazanfari MJ, Hosseini SJ, et al. Near‐death experiences of burn survivors: an important yet challenging issue. Burns. 2023. In press. [DOI] [PubMed] [Google Scholar]
- 65. Argenta A, Satish L, Gallo P, Liu F, Kathju S. Local application of probiotic bacteria prophylaxes against sepsis and death resulting from burn wound infection. PLoS One. 2016;11(10):e0165294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Abasgholizade M, Fozouni L. Antagonistic effect of probiotics on drug resistant Pseudomonas aeruginosa isolated from burn wound infection. Int J Hosp Res. 2017;6(2):104‐110. [Google Scholar]
- 67. DehghanZadeh Z, Koupaei M, Ghorbani Z, Saderi H, Marashi SMA, Owlia P. Inhibitory effect of Saccharomyces cerevisiae supernatant and lysate on expression of lasB and apl genes of Pseudomonas aeruginosa . Gene Rep. 2021;24:101247. [Google Scholar]
- 68. Shams Eldeen MA, Elnahal A, Ads A, Elsawaf M, Samy SM. Effect of Lactobacillus plantarum on virulence factors of Pseudomonas aeruginosa isolated from wound infection. Microb Infect Dis. 2021;2(4):790‐796. [Google Scholar]
- 69. Alexandre Y, Le Berre R, Barbier G, Le Blay G. Screening of Lactobacillus spp. for the prevention of Pseudomonas aeruginosa pulmonary infections. BMC Microbiol. 2014;14(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Al‐Malkey MK, Ismeeal MC, Al‐Hur FJA, Mohammed SW, Nayyef HJ. Antimicrobial effect of probiotic Lactobacillus spp. on Pseudomonas aeruginosa . J Contemp Med Sci. 2017;3(10):218‐223. [Google Scholar]
- 71. Abootaleb M, Bandari NM, Soleimani NA. Interference of Lactobacillus casei with Pseudomonas aeruginosa in the treatment of infected burns in Wistar rats. Iran J Basic Med Sci. 2021;24(2):143‐149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Shokri D, Khorasgani MR, Mohkam M, Fatemi SM, Ghasemi Y, Taheri‐Kafrani A. The inhibition effect of lactobacilli against growth and biofilm formation of Pseudomonas aeruginosa . Probiot Antimicrob Prot. 2018;10(1):34‐42. [DOI] [PubMed] [Google Scholar]
- 73. Sharma V, Harjai K, Shukla G. Effect of bacteriocin and exopolysaccharides isolated from probiotic on P. aeruginosa PAO1 biofilm. Folia Microbiol. 2018;63(2):181‐190. [DOI] [PubMed] [Google Scholar]
- 74. Valdez J, Peral M, Rachid M, Santana M, Perdigon G. Interference of Lactobacillus plantarum with Pseudomonas aeruginosa in vitro and in infected burns: the potential use of probiotics in wound treatment. Clin Microbiol Infect. 2005;11(6):472‐479. [DOI] [PubMed] [Google Scholar]
- 75. Chatterjee M, Anju C, Biswas L, Kumar VA, Mohan CG, Biswas R. Antibiotic resistance in Pseudomonas aeruginosa and alternative therapeutic options. Int J Med Microbiol. 2016;306(1):48‐58. [DOI] [PubMed] [Google Scholar]
- 76. Rashno Taee S, Khansarinejad B, Abtahi H, Najafimosleh M, Ghaznavi‐Rad E. Detection of algD, oprL and exoA genes by new specific primers as an efficient, rapid and accurate procedure for direct diagnosis of Pseudomonas aeruginosa strains in clinical samples. Jundishapur J Microbiol. 2014;7(10):e13583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lenzmeier TD, Mudaliar NS, Stanbro JA, et al. Application of Lactobacillus gasseri 63 AM supernatant to Pseudomonas aeruginosa‐infected wounds prevents sepsis in murine models of thermal injury and dorsal excision. J Med Microbiol. 2019;68(10):1560‐1572. [DOI] [PubMed] [Google Scholar]
- 78. Fijan S, Kocbek P, Steyer A, Vodičar PM, Strauss M. The antimicrobial effect of various single‐strain and multi‐strain probiotics, dietary supplements or other beneficial microbes against common clinical wound pathogens. Microorganisms. 2022;10(12):2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kiymaci ME, Altanlar N, Gumustas M, Ozkan SA, Akin A. Quorum sensing signals and related virulence inhibition of Pseudomonas aeruginosa by a potential probiotic strain's organic acid. Microb Pathog. 2018;121:190‐197. [DOI] [PubMed] [Google Scholar]
- 80. Fangous M‐S, Gosset P, Galakhoff N, et al. Priming with intranasal lactobacilli prevents Pseudomonas aeruginosa acute pneumonia in mice. BMC Microbiol. 2021;21(1):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Díaz MA, González SN, Alberto MR, Arena ME. Human probiotic bacteria attenuate Pseudomonas aeruginosa biofilm and virulence by quorum‐sensing inhibition. Biofouling. 2020;36(5):597‐609. [DOI] [PubMed] [Google Scholar]
- 82. Barzegari AA, Hashemzaei M, Majdani R, Alihemmati A‐R. Effects of topical treatment of second‐degree burn wounds with Lactobacillus acidophilus on the wound healing process in male rats. Pharm Biomed Res. 2017;3(3):23‐30. [Google Scholar]
- 83. Vuotto C, Longo F, Donelli G. Probiotics to counteract biofilm‐associated infections: promising and conflicting data. Int J Oral Sci. 2014;6(4):189‐194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Aminnezhad S, Kermanshahi RK, Ranjbar R. Evaluation of synergistic interactions between cell‐free supernatant of Lactobacillus strains and amikacin and genetamicin against Pseudomonas aeruginosa . Jundishapur J Microbiol. 2015;8(4):e16592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Haghighatafshar H, Talebi R, Tukmechi A. The effect of bacteriocin isolated from Lactobacillus rhamnosus on Pseudomonas aeruginosa lipopolysaccharides. Avicenna J Clin Microbiol Infect. 2021;8(2):45‐50. [Google Scholar]
- 86. Salman JAS, Kareem AJ. Antibacterial and anti virulence factors of purified dextran from Lactobacillus gasseri against Pseudomonas aeruginosa . Jordan J Biol Sci. 2021;14(1):191‐197. [Google Scholar]
- 87. Alexandre Y, Le Blay G, Boisramé‐Gastrin S, et al. Probiotics: a new way to fight bacterial pulmonary infections? Med Mal Infect. 2014;44(1):9‐17. [DOI] [PubMed] [Google Scholar]
- 88. Ramos AN, Sesto Cabral ME, Noseda D, Bosch A, Yantorno OM, Valdez JC. Antipathogenic properties of Lactobacillus plantarum on P seudomonas aeruginosa: the potential use of its supernatants in the treatment of infected chronic wounds. Wound Repair Regen. 2012;20(4):552‐562. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used during the current study are available from the corresponding author upon request.


