Abstract
BACKGROUND
Regulated cell death is a fundamental component of numerous physiological processes; spanning from organogenesis in utero, to normal cell turnover during adulthood, as well as the elimination of infected or damaged cells throughout life. Quality control through regulation of cell death pathways is particularly important in the germline, which is responsible for the generation of offspring. Women are born with their entire supply of germ cells, housed in functional units known as follicles. Follicles contain an oocyte, as well as specialized somatic granulosa cells essential for oocyte survival. Follicle loss—via regulated cell death—occurs throughout follicle development and life, and can be accelerated following exposure to various environmental and lifestyle factors. It is thought that the elimination of damaged follicles is necessary to ensure that only the best quality oocytes are available for reproduction.
OBJECTIVE AND RATIONALE
Understanding the precise factors involved in triggering and executing follicle death is crucial to uncovering how follicle endowment is initially determined, as well as how follicle number is maintained throughout puberty, reproductive life, and ovarian ageing in women. Apoptosis is established as essential for ovarian homeostasis at all stages of development and life. However, involvement of other cell death pathways in the ovary is less established. This review aims to summarize the most recent literature on cell death regulators in the ovary, with a particular focus on non-apoptotic pathways and their functions throughout the discrete stages of ovarian development and reproductive life.
SEARCH METHODS
Comprehensive literature searches were carried out using PubMed and Google Scholar for human, animal, and cellular studies published until August 2022 using the following search terms: oogenesis, follicle formation, follicle atresia, oocyte loss, oocyte apoptosis, regulated cell death in the ovary, non-apoptotic cell death in the ovary, premature ovarian insufficiency, primordial follicles, oocyte quality control, granulosa cell death, autophagy in the ovary, autophagy in oocytes, necroptosis in the ovary, necroptosis in oocytes, pyroptosis in the ovary, pyroptosis in oocytes, parthanatos in the ovary, and parthanatos in oocytes.
OUTCOMES
Numerous regulated cell death pathways operate in mammalian cells, including apoptosis, autophagic cell death, necroptosis, and pyroptosis. However, our understanding of the distinct cell death mediators in each ovarian cell type and follicle class across the different stages of life remains the source of ongoing investigation. Here, we highlight recent evidence for the contribution of non-apoptotic pathways to ovarian development and function. In particular, we discuss the involvement of autophagy during follicle formation and the role of autophagic cell death, necroptosis, pyroptosis, and parthanatos during follicle atresia, particularly in response to physiological stressors (e.g. oxidative stress).
WIDER IMPLICATIONS
Improved knowledge of the roles of each regulated cell death pathway in the ovary is vital for understanding ovarian development, as well as maintenance of ovarian function throughout the lifespan. This information is pertinent not only to our understanding of endocrine health, reproductive health, and fertility in women but also to enable identification of novel fertility preservation targets.
Keywords: fertility, ovary, oocyte, granulosa cell, regulated cell death, apoptosis, necroptosis, autophagy, pyroptosis, parthanatos
Graphical Abstract
Graphical Abstract.
Although apoptosis has well-established roles in regulating ovarian follicle number across the lifespan, recent evidence suggests autophagic cell death, necroptosis, pyroptosis, and parthanatos also contribute.
Introduction
Regulated cell death plays an integral role in tissue and organ differentiation during foetal development, and in the maintenance of cell turnover throughout subsequent stages of life. These cell death pathways are critical for responding to toxic insults or microbial infection, while dysfunctional cell death signalling can be implicated in the development of some disease states in humans (Kist and Vucic, 2021). Accordingly, some cell death regulators represent viable therapeutic targets. The ovary houses the female germline in the form of oocytes, which are encapsulated by somatic granulosa and theca cells to form ovarian follicles. Women are born with their lifetime supply of follicles termed the ‘ovarian reserve’. Therefore, maintaining the quality of the reserve of long-lived oocytes is crucial to ensure the generation of healthy, viable children (Kerr et al., 2012a).
Across the reproductive lifespan, regulated cell death plays fundamental roles during follicle formation, follicle development, ovulation, and the elimination of damaged oocytes. Therefore, understanding the specific pathways and key factors involved in the regulation of oocyte attrition (death of the oocyte specifically) and follicle atresia (death of the whole follicle, oocyte, and supporting somatic cells) has been the topic of intensive study over the past several decades. Animal models have contributed vital insights since the availability of human tissue for study is limited.
During foetal development, there is a considerable oversupply of female gametes, which are initially generated by foetal germ cell proliferation before entry into meiosis. However, this supply is significantly depleted by extensive oogonial and oocyte loss during follicle formation (Kerr et al., 2013; Findlay et al., 2015). Oogonia and oocyte survival during ovarian development is dependent on the activation or suppression of cell death signals. These signals dictate the size and quality of the follicle pool that women will be endowed with, which will ultimately define their lifetime fertility (Kerr et al., 2013; Findlay et al., 2015). The process of follicle development (folliculogenesis) then commences, during which a subset of immature primordial follicles is activated to develop and mature through discrete stages of growth (pre-antral, antral, and pre-ovulatory), ultimately producing a single mature ovulatory oocyte with each menstrual cycle in women. During puberty, there is a surge in follicle atresia, which is thought to be predominately hormonally induced, though the exact reasons for this remain unknown (Liew et al., 2017). Folliculogenesis occurs throughout reproductive life and, like follicle formation, is subject to a significant amount of redundancy, since more follicles are activated than will actually be ovulated. Indeed, over 99% of activated follicles will undergo natural atresia (Wallace and Kelsey, 2010; Pelosi et al., 2015).
In addition to natural atresia, exposure to endogenous and exogenous insults that may be detrimental to oocyte quality can readily trigger regulated cell death pathways and follicle loss. Although, in some cases, this might be necessary in order to maintain the integrity of the female germline (Winship et al., 2018). For example, it is well-established that exposure to ionizing radiation, certain chemotherapies, and various environmental toxicants can induce DNA damage and significantly alter the balance of follicle atresia versus survival and maturation (Oktem and Oktay, 2007; Tatone et al., 2008; Soleimani et al., 2011; Winship et al., 2018). Additionally—depending on the severity of damage—exposure to such agents can completely ablate the follicle pool, leading to permanent infertility and premature menopause (Kerr et al., 2012a; Titus et al., 2021). Thus, understanding the precise mechanisms of regulated cell death that occur within ovarian follicles in response to various exogenous insults has important implications for developing effective fertility preservation strategies.
In this review, we provide a comprehensive overview of the most recent literature that identifies cell death pathway regulators in the ovary, highlighting apoptotic and non-apoptotic pathways, and their functions throughout the discrete stages of ovarian development and reproductive life.
Methods
Comprehensive literature searches using PubMed and Google Scholar were conducted for this review to identify peer-reviewed English publications for human, animal, and cellular studies published until August 2022. The search included keywords in the following areas: oogenesis, follicle formation, follicle atresia, oocyte loss, oocyte apoptosis, regulated cell death in the ovary, non-apoptotic cell death in the ovary, premature ovarian insufficiency (POI), primordial follicles, oocyte quality control, granulosa cell death, autophagy in the ovary, autophagy in oocytes, necroptosis in the ovary, necroptosis in oocytes, pyroptosis in the ovary, pyroptosis in oocytes, parthanatos in the ovary, and parthanatos in oocytes.
Types of regulated cell death that occur within the ovary
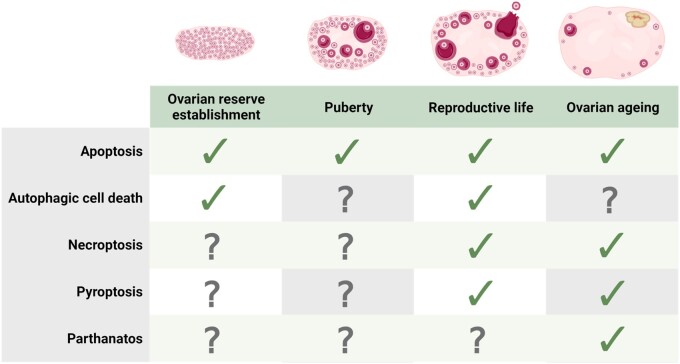
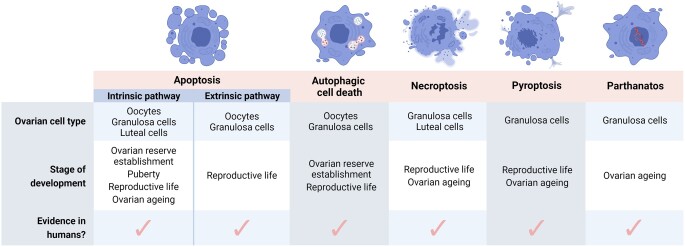
Regulated cell death refers to the programmed induction of cell death by specific molecular pathways. In the ovary, apoptosis has been the most widely investigated regulated cell death pathway to date (Liew et al., 2016; Marcozzi et al., 2018; Li, 2021), with new insights into the mechanisms of apoptotic cell death within the ovary published regularly (Table I). However, recent evidence indicates that other cell death pathways—including autophagy and autophagic cell death, necroptosis, pyroptosis, and parthanatos—may also play important roles in regulating ovarian function across the lifespan (Fig. 1; Table II). The classification of these various pathways differs in their gross cellular morphology, pathophysiological relevance, and by the specific signal transduction molecules that activate and execute the cell’s demise. However, it is important to appreciate that additional regulated cell death pathways exist in other tissues and cell types (reviewed in detail by Galluzzi et al., 2018).
Table I.
Recent insights into the mechanisms of apoptotic cell death within the ovary.
| Species | Study type | Factor(s) | Developmental stage | Ovarian cell type | Study design | Main findings | Reference |
|---|---|---|---|---|---|---|---|
| Human | In vivo | Leucyl-tRNA synthetase 2 (LARS2) | Follicle atresia | Granulosa cells | Analysis of LARS2 expression in human granulosa cells derived from patients with premature ovarian insufficiency (POI). | LARS2 expression is decreased in granulosa cells of POI patients. Knockdown of LARS2 induces granulosa cell apoptosis and impairs mitochondrial function, by increasing reactive oxygen species (ROS) levels. | (Feng et al., 2022) |
|
| |||||||
| GnRH agonists | Oocyte maturation |
|
Analysis of follicular fluid collected from human follicles. Mural granulosa cells and luteal cells isolated from follicular fluid. | Significantly increased apoptosis in cumulus oocyte complexes (COCs) from women treated with GnRH agonists. Suggests that GnRH triggers could impair follicle maturation and trigger corpus luteum regression. | (Gonen et al., 2021) | ||
|
| |||||||
| In vitro | Sirtuin-1 (SIRT1) | Follicle atresia | Granulosa cells | Analysis of primary and immortalized human granulosa cells treated with SRT2104 (a SIRT1 activator). | SRT2104 significantly increased the number of apoptotic cells, as well as elevating pro-apoptotic cleaved caspase-3 and cleaved poly-(ADP-ribose) polymerase (PARP) levels, suggesting SIRT1 is involved in apoptosis within the ovary. | (Sapuleni et al., 2022) | |
|
| |||||||
| Anandamide (AEA) |
|
Granulosa cells | In vitro culture of human immortalized granulosa cells (COV434) and human granulosa cells ± AEA. | AEA reduces cell viability and induces granulosa cell apoptosis via extrinsic pathway. Suggests balance of endocannabinoids is crucial for normal follicle development. | (Costa et al., 2021) | ||
|
| |||||||
| Phosphate and tensin homolog (PTEN) | Oocyte maturation | Granulosa cells | Analysis of PTEN expression in human granulosa cells. Knockdown of PTEN in vitro using shRNA. | PTEN expression promotes granulosa cell apoptosis. Knockdown of PTEN significantly reduces granulosa cell apoptosis. | (Yao et al., 2021) | ||
|
| |||||||
| Cow | In vitro | Bone morphogenic protein (BMP) 4 | Follicle development | Granulosa cells | Analysis of the expression and function of BMP4 in cultured bovine cumulus cells. | Knockdown of BMP4 induced apoptosis and cell-cycle arrest in bovine cumulus cells. BMP4 is an important regulator of granulosa cell proliferation via regulation of apoptosis. | (Tian et al., 2022) |
|
| |||||||
| Pig | In vitro |
|
Oocyte maturation | Granulosa cells | In vitro culture of porcine COCs and granulosa cells ± cortisol and/or FSH. | Cortisol induces granulosa cell apoptosis. FSH prevents this cortisol-induced apoptosis. | (Nakanishi et al., 2021) |
| Pig |
|
Oocyte maturation | Granulosa cells | In vitro analysis of cultured porcine granulosa cells collected from antral follicles. | Hx prevents the G2-M transition in porcine granulosa cells, inducing cell cycle arrest and apoptosis. Oocyte factors GDF9 and BMP15 counteract this effect. Suggests counterbalance of intrafollicular factors is important for regulating cell cycle progression of granulosa cells. | (Li et al., 2020) | |
|
| |||||||
| Mouse | In vivo | Lon protease 1 (LONP1) |
|
Oocytes | Characterization of oocyte-specific conditional Lonp1–/– reproductive phenotype in mice. | Conditional loss of Lonp1 in oocytes significantly depletes primordial and growing follicles, leading to infertility. Lonp1 is critical for oocyte survival, due to its suppression of apoptosis inducing factor mitochondria-associated 1 (AIFM1) translocation to the nucleus. | (Sheng et al., 2022) |
|
| |||||||
| Complement 1Q-like protein (C1QL1) |
|
Granulosa cells | Characterization of reproductive phenotype of C1QL1-deficient mice (using C1QL1 antiserum). | Loss of C1QL1 increased granulosa cell apoptosis and antral follicle atresia. C1QL1 has important functions in regulating granulosa cell apoptosis, via AKT/mammalian target of rapamycin (mTOR) signalling. | (Lu et al., 2022) | ||
|
| |||||||
|
Ovarian ageing |
|
Treatment of ageing mice with GH. Analysis of follicle counts and superovulation. | GH treatment decreased oocyte apoptosis and improved mature oocyte quality. Suggests decreased GH levels and associated increase in c-Jun N-terminal Kinase (JNK) signalling mediates the age-related decline in oocyte quality. | (Liu et al., 2021) | ||
|
| |||||||
|
Ovarian reserve establishment | Primordial follicles | Selective deletion of Trp63 exon 13 (Δ13p63) in mice, which deletes the TAp63α isoform only. | Δ13p63+/– mice are completely infertile and have almost complete depletion of primordial follicles by postnatal day (PN) 10 via intrinsic apoptosis. Shows that integrity of the p63 C-terminus is critical for oocyte development and survival. | (Lena et al., 2021) | ||
|
| |||||||
|
Reproductive life |
|
Characterization of oocyte-and granulosa cell-specific conditional Wntless–/– reproductive phenotype in mice | Granulosa cell-specific conditional Wntless–/–mice were subfertile and experienced recurrent miscarriage. Suggests deletion of Wntless impairs luteinization and induces granulosa cell apoptosis via intrinsic pathway. | (Cheng et al., 2020) | ||
| Cortisol | Oocyte maturation |
|
Treatment of wild-type and tumour necrosis factor alpha (TNF-α) deficient mice with cortisol in vivo. | Cortisol impaired oocyte competence, increased oxidative stress, and induced mural granulosa cell apoptosis via extrinsic pathway. | (Yuan et al., 2020) | ||
|
| |||||||
| Specificity protein 1 (SP1) | Ovarian reserve establishment | Primordial follicles | Global and granulosa cell-specific Sp1 knockdown in mice. | Knockdown of Sp1, especially in granulosa cells, suppresses nest breakdown, oocyte apoptosis and formation of primordial follicles. | (Cai et al., 2020) | ||
|
| |||||||
| B lymphoma Mo-MLV insertion region 1 (BMI1) |
|
|
Characterization of Bmi–/– mouse reproductive phenotype | Complete infertility, disrupted estrous cyclicity and delayed onset of puberty in Bmi–/– mice. Significant reduction of primordial follicles and mature oocytes. Increased granulosa cell apoptosis via intrinsic pathway and mitochondrial dysfunction. | (Wang et al., 2019) | ||
|
| |||||||
| Ex vivo |
|
Follicle atresia | Primordial follicles |
|
TNF-α significantly depletes primordial follicle numbers in wild-type, but not Bid–/– ovaries, suggesting that TNF-α can directly induce primordial follicle atresia via the extrinsic apoptosis pathway. | (Winship et al., 2022) | |
|
| |||||||
| In vitro | Orexin-A (OXA) |
|
Granulosa cells | Knockdown of OXA receptor 1 (OXR1) in mouse primary granulosa cells. | OXA (a neuropeptide) and OXR1 are expressed in mouse primary granulosa cells. OXA regulates granulosa cell proliferation and apoptosis in vitro via the AKT/ERK signalling pathway, thus may have roles in regulating follicle growth and atresia. | (Safdar et al., 2021) | |
|
| |||||||
| Chemokine (C–C motif) ligand 5 (CCL5) |
|
Granulosa cells | Analysis of cultured mouse ovarian follicles and granulosa cells. | CCL5 impairs oocyte maturation and promotes granulosa cell apoptosis in vitro. Suggests CCL5 secretion by theca-interstitial cells may impair follicle development and maturation during ovarian ageing. | (Shen et al., 2019) | ||
|
| |||||||
| Caenorhabditis elegans | In vivo | DNA topoisomerase 3 (TOP3) | Oocyte quality control and ovarian reserve maintenance | Oocytes | Analysis of top-3 C. elegans mutants. | Loss of top-3 impairs the ability to eliminate defective oocytes, suggesting that top-3 is critical for oocyte quality control via intrinsic apoptosis. | (Dello Stritto et al., 2021) |
Figure 1.
Types of cell death active within the ovary throughout various stages of development. The regulated cell death pathways apoptosis (intrinsic and extrinsic), autophagic cell death, necroptosis, pyroptosis, and parthanatos are all active within the ovary in numerous species, including humans. The ovarian cell type and stage of development in which evidence has been published is summarized. Figure created using BioRender.
Table II.
Emerging evidence suggesting the contribution of other regulated cell death pathways to ovarian function.
| Cell death pathway | Species | Study type | Factor(s) | Developmental stage | Ovarian cell type | Study design | Main findings | Reference |
|---|---|---|---|---|---|---|---|---|
| Autophagic cell death | Human | In vitro | – |
|
Granulosa cells | Human granulosa cells treated with apoptosis-inducing substances in vitro. Analysis of autophagy and phagocytosis markers. | Granulosa cells ingest and destroy apoptotic oocytes via autophagy-assisted phagocytosis | (Yefimova et al., 2020) |
|
| ||||||||
| Cow | In vitro | FSH | Oocyte maturation | Granulosa cells | Bovine granulosa cells treated with increasing doses of FSH in vitro. | High doses of FSH induce autophagy in bovine granulosa cells. Suggests why aggressive FSH stimulation in patients leads to poor oocyte quality and embryo development. | (Tang et al., 2021a) | |
|
| ||||||||
| Pig | In vivo | Light chain 3B (LC3B) |
|
|
Analysis of autophagy and apoptosis markers in granulosa cells, cumulus cells, and oocytes isolated from porcine cumulus oocyte complexes (COCs). | Significant increase in abundance of LC3B-II protein in granulosa cells, cumulus cells and oocytes from both early and late stage atretic follicles. Suggests that growing follicle atresia is regulated by both apoptosis and autophagy of granulosa cells. | (Gioia et al., 2019) | |
|
| ||||||||
| Rat | In vivo | Beclin 1 (BECN1) | Follicle atresia | Oocytes | Analysis of BECN1 levels in pre-pubertal, juvenile, and adult rat ovaries. | In atretic oocytes, high levels of BECN1 are coupled with high levels of caspase-3, BAX, and BAK. Suggests that BECN1, a pro-autophagic protein, promotes apoptosis of oocytes. | (Escobar et al., 2019) | |
|
| ||||||||
|
Hypoxia-inducible factor (HIF)-1α | Corpus luteum formation | Granulosa cells | Rats treated in vivo with a HIF-1α inhibitor. In vitro analysis of cultured rat granulosa cells. | HIF-1α plays a crucial role in regulating granulosa cell luteinization and subsequent early corpus luteum development. Inhibition of HIF-1α increased apoptosis in early corpora lutea. | (Tang et al., 2021b) | ||
|
| ||||||||
| Mouse | In vivo | – | Ovarian reserve establishment | Primordial follicles | Inhibition of autophagy using 3-methyladenine in perinatal mice. | Active autophagy observed in ovaries from 16.5 days post coitum (dpc) to postnatal day (PN) 3. Inhibition of autophagy increased number of cyst oocytes and delayed follicle formation. Suggests autophagy assists in germ cell cyst breakdown and primordial follicle assembly. | (Zhihan et al., 2019) | |
|
| ||||||||
| Ex vivo | Lysine-specific demethylase 1 (LSD1) | Ovarian reserve establishment | Primordial follicles | Ex vivo culture of perinatal mouse ovaries, with Lsd1 either knocked down or overexpressed using specific siRNAs. | LSD1 is highly expressed in mouse foetal ovaries, but sharply reduces from 18.5 dpc onwards. Suggests that, via regulation of autophagy, LSD1 contributes to the initiation of apoptosis during ovarian reserve establishment. | (He et al., 2020) | ||
| Necroptosis | Human | In vivo | Phosphoglycerate translocase 5 (PGAM5) | Ovarian ageing | Granulosa cells | Analysis of PGAM5 expression in human cumulus cells. | PGAM5 expression in human cumulus cells increases with advancing age and is associated with decreased mitochondrial function, which may implicate a role for necroptosis in the process of ovarian ageing. | (Li et al., 2022) |
|
| ||||||||
| In vitro | Sirtuin-1 (SIRT1) | Follicle atresia | Granulosa cells | Analysis of primary and immortalized human granulosa cells treated with SRT2104 (a SIRT1 activator) and Nec-1 (a necroptosis inhibitor). | SRT2104 significantly increased the number of necrotic cells, as well as elevating pro-necroptotic receptor-interacting serine/threonine-protein kinase (RIPK) 1 and mixed lineage kinase domain-like pseudokinase (MLKL) protein levels. Nec-1 attenuated RIPK1 and MLKL levels, suggesting SIRT1 is involved in necroptosis within the ovary. | (Sapuleni et al., 2022) | ||
|
| ||||||||
| Cow | In vivo | RIPK1 and RIPK3 | Oocyte maturation | Granulosa cells | Analysis of mRNA expression of RIPK1 and RIPK3 in granulosa and theca cells derived from healthy and atretic bovine follicles. | Suggests that both apoptosis and necroptosis occur within granulosa cells of dominant follicles undergoing luteinization. | (McEvoy et al., 2021) | |
|
| ||||||||
| Pig | In vivo | Chemerin |
|
Luteal cells | High throughput sequencing of the transcriptome of cultured mid-luteal stage porcine luteal cells. | Chemerin (an adipokine) interacts strongly with necroptosis-associated genes during the mid-luteal phase, suggesting a potential role for necroptosis (in conjunction with apoptosis) in facilitating corpus luteum regression. | (Makowczenko et al., 2022) | |
|
| ||||||||
| Pyroptosis | Human | In vivo | Gasdermin family members (GSDMs) | – | – | Analysis of expression of GSDMs in human ovarian tissue from patients with serous ovarian cancer and healthy counterparts. | Many GSDMs, including gasdermin D (GSDMD—a key pore-forming protein involved in pyroptosis) are expressed in normal ovarian tissue. | (Berkel and Cacan, 2021) |
|
| ||||||||
| Cow |
|
Non-esterified fatty acids (NEFAs) | Follicle atresia | Granulosa cells | Analysis of serum and cultured granulosa cells obtained from bovine ovaries. | NEFAs induced pyroptosis and inflammation of granulosa cells in vitro, as evidenced by increased NLR family pyrin domain containing 3 (NLRP3), toll-like receptor 4 (TLR4), caspase-1, and interleukin-1β expression. | (Wang et al., 2020) | |
| Rat |
|
Polystyrene microplastics (PS MPs) | Follicle atresia | Granulosa cells | Analysis of serum, ovaries and cultured primary granulosa cells in rats. | PS MPs activated the NLRP3/caspase-1 signalling pathway in ovarian granulosa cells possibly triggered by oxidative stress. | (Hou et al., 2021) | |
|
| ||||||||
| Mouse | In vivo | NLRP3 inflammasome | Ovarian ageing | Follicles | Characterization of Nlrp3–/– mouse phenotype across the reproductive lifespan. | Although not directly assessed, both publications suggest that pyroptosis is contributing to age-related follicle depletion in mice, via activation of the NLRP3 inflammasome. | ||
|
| ||||||||
| Parthanatos | Human | In vitro | Poly(ADP-ribose) (PAR) | Oocyte maturation | Cumulus granulosa cells | Oocytes collected from normal and diminished ovarian reserve (DOR) patients. Cumulus cells isolated and cultured. | Increased PAR expression in cumulus cells of DOR patients. Suggests poly[ADP-ribose] (PAR) polymerase 1 (PARP-1)-dependent cell death may contribute to diminished ovarian reserve. | (Batnasan et al., 2020) |
Apoptosis
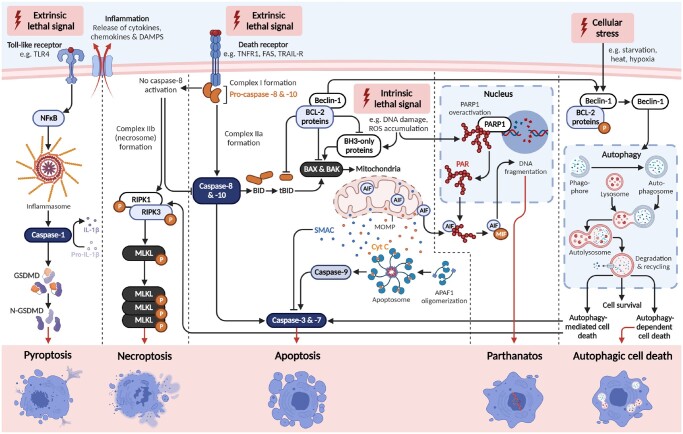
Apoptosis is the programmed, controlled death of a cell; which involves degradation and fragmentation of protein and DNA, and engulfment of the collapsed cell by neighbouring cells and/or phagocytes in a non-inflammatory manner. It occurs in all multicellular organisms throughout life from foetal development onwards and is an essential homeostatic mechanism to maintain healthy cell populations in tissues and organs. There are two major apoptotic pathways: the intrinsic and extrinsic pathways (Fig. 2). The intrinsic (mitochondrial) pathway is activated from within the cell and is predominately regulated by mitochondria. On the other hand, the extrinsic (death receptor) pathway is triggered from outside the cell, typically in response to conditions and factors within the extracellular environment.
Figure 2.
Overview of regulated cell death pathways. A summary of each of the well-described regulated cell death pathways—apoptosis (intrinsic and extrinsic), autophagic cell death, necroptosis, pyroptosis, and parthanatos. Intrinsic apoptosis: After an intrinsic lethal signal occurs (e.g. DNA damage), BH3-only proteins activate BAX and BAK either directly, or indirectly by binding and inhibiting BCL-2 proteins. Mitochondrial outer membrane permeabilization (MOMP) then occurs, which releases cytochrome C (Cyt C) and SMAC, the latter of which can inhibit apoptosis. The apoptosome is then formed, leading to caspase-9 activation, subsequent caspase-3 and -7 activation, and initiation of apoptosis. Extrinsic apoptosis: Once death receptors (e.g. TNFR1, FAS, or TRAIL-R) detect an extrinsic lethal signal, this receptor associates with pro-caspase-8 and -10 to form complex I. Complex IIa is subsequently formed, which leads to caspase-8 and -10 activation. Apoptosis is then initiated either directly, via direct cleavage of caspase-3 and -7; or indirectly, via cleavage of BID into tBID and subsequent activation of BAX and BAK. Necroptosis: Following an extrinsic lethal signal and in the absence of caspase-8 activation, complex IIb (i.e. the necrosome) is formed. This leads to phosphorylation of receptor-interacting serine/threonine-protein kinase (RIPK) 1 and 3, which phosphorylate and activate mixed lineage kinase domain-like pseudokinase (MLKL). MLKL then forms a complex, resulting in release of cytokines, chemokines, and damage-associated molecular patterns (DAMPS). Ultimately, this results in inflammation and necroptosis of the cell. Pyroptosis: Once toll-like receptors (e.g. TLR4) detect an extrinsic lethal signal, nuclear factor kappa B (NF-κB) signalling is activated. This results in inflammasome formation and subsequent caspase-1 activation. Then, pro-IL-1β is converted into IL-1β, and gasdermin D (GSDMD) is cleaved into N-GSDMD fragments. This leads to inflammation and pyroptosis of the cell. Parthanatos: Once an intrinsic lethal signal occurs (e.g. excessive reactive oxygen species accumulation), poly[ADP-ribose] polymerase 1 (PARP-1) becomes activated. If PARP-1 overactivation occurs, this can lead to accumulation of PAR polymer and translocation of apoptosis inhibitory factor (AIF) from mitochondria. AIF forms a complex with macrophage migration inhibitory factor (MIF), which re-enters the nucleus. Ultimately, this leads to DNA fragmentation and parthanatos of the cell. Autophagic cell death: Beclin-1 normally exists in a complex with BCL-2 proteins. Once these have been phosphorylated and inactivated, free Beclin-1 can then initiate autophagy. Autophagy involves fusion of the autophagosome and lysosome to form the autolysosome, which then degrades and recycles intracellular components. This can lead to cell survival, but sometimes can cause autophagy-mediated cell death (by activating either apoptosis or necroptosis) or autophagy-dependent cell death (i.e. cell death without apoptosis or necroptosis). Figure created using BioRender.
Intrinsic apoptosis
The intrinsic apoptosis pathway is initiated by a variety of non-receptor-mediated microenvironmental perturbations, including growth factor withdrawal, DNA damage, endoplasmic reticulum stress, reactive oxygen species (ROS) overload, replication stress, and microtubular alterations or mitotic defects, among others (Elmore, 2007; Li and Yuan, 2008; Suen et al., 2008; Wang and Youle, 2009). These stimuli produce intracellular signals that cause disruptions to the mitochondrial membrane, which result in mitochondrial inner and outer membrane permeabilization, loss of mitochondrial transmembrane potential, and release of normally sequestered pro-apoptotic proteins from the intermembrane space into the cytosol. This release of pro-apoptotic proteins is considered the ‘point of no return’ in apoptosis, after which cytochrome c release, caspase activation (predominately caspase-3), formation of the apoptosome, and death of the cell will occur (Aubrey et al., 2018).
The intrinsic apoptosis pathway is controlled by members of the B cell lymphoma 2 (BCL-2) family of proteins. These can be divided into three subgroups based on their structure and function: the anti-apoptotic BCL-2 proteins, including BCL-2, BCL-XL, BCL-W, MCL-1, and A1; the pro-apoptotic proteins, BAX, BAK, and BOK; and the BH3-only proteins PUMA, NOXA, BH3 interacting-domain death agonist (BID), BAD, BIM, BIK, HRK, and BCL-2 modifying factor (BMF). The BH3-only proteins are responsible for sensing apoptotic signals and transmitting them to other BCL-2 family members to ultimately trigger the apoptotic cascade. They do this by binding and inhibiting the core anti-apoptotic BCL-2 proteins, leading to conversion of BAX, BAK, and BOK from inert monomers into membrane-permeabilizing oligomers (Moldoveanu and Czabotar, 2020). Once permeabilized, apoptogenic factors, such as cytochrome c, are released from the mitochondrial intermembrane space and trigger caspase activation.
Extrinsic apoptosis
The extrinsic apoptotic pathway can be activated by two types of plasma membrane receptors: death receptors, which are activated by cognate ligand binding (Guicciardi and Gores, 2009); and dependence receptors, which are activated when specific ligands drop below a certain threshold (Goldschneider and Mehlen, 2010). The most widely characterized death receptors include, but are not limited to, FAS cell surface death receptors and the tumour necrosis factor (TNF) receptor superfamily members (Wajant and Siegmund, 2019). Briefly, binding of the death receptor ligand to the receptor allows the assembly of the death-inducing signalling complex that regulates the activation of pro-caspase-8 and -10.
Although the mitochondrial pathway is strongly associated with intrinsic apoptosis; in certain cell types, the extrinsic pathway can crosstalk with the intrinsic pathway through caspase-8-mediated proteolytic cleavage of tBID to BID, which triggers the release of apoptogenic factors to activate BAX and induce apoptosis (Cui et al., 2016).
Autophagy and autophagic cell death
Derived from the Greek language, meaning ‘self-eating’, autophagy is a tightly-regulated process whereby cells degrade and recycle their own cytosolic components inside lysosomes, which can lead to cell death (Galluzzi et al., 2018; Schwartz, 2021). Unlike apoptosis, this form of regulated cell death occurs in the absence of chromatin condensation and phagocytes. Autophagic cell death manifests in the accumulation of large numbers of autophagic vesicles containing cytoplasmic material for degradation by lysosomes, and results in early degradation of organelles and late degradation of cytoskeleton, which is the reverse for apoptotic cells (Cuervo, 2004; Thorburn, 2008).
Autophagy is mediated by dozens of autophagy-related (ATG) proteins that regulate expanding ‘isolation membranes’, which encapsulate and enclose proteins/organelles into a double-membrane structure called the autophagosome (Li et al., 2021b). These autophagosomes then fuse with liposomes to degrade the internal components. Once fused, acidic hydrolases in the lysosome can degrade the autophagic cargos, and salvaged nutrients are released to the cytoplasm to be recycled by cells. Genetic models have demonstrated that some autophagic machinery is essential for regulated cell death (e.g. ATG1, reviewed by Schwartz, 2021); however, it has been suggested that it might be more appropriate to name the process ‘autophagy-mediated cell death’ (Kroemer and Levine, 2008). Although, more recent literature suggests that autophagy-dependent cell death, that is independent of apoptosis or other regulated cell death pathways, can occur (Bialik et al., 2018; Denton and Kumar, 2019; Kriel and Loos, 2019) (Fig. 2). Indeed, there is strong evidence that apoptotic and autophagic machineries are highly interconnected during developmental regulated cell death (Zhang and Baehrecke, 2015).
Necroptosis
Necrosis is morphologically distinct from apoptosis and characterized by a gain in cell volume, organelle swelling, plasma membrane rupture, and loss of intracellular contents (Galluzzi et al., 2018). Unlike apoptosis, necrosis provokes an inflammatory response by spilling the cell’s cytosolic constituents into the extracellular space through the damaged plasma membrane. During apoptosis, however, these products are safely isolated by membranes and then consumed by phagocytes. Necroptosis is a programmed, regulated form of necrosis that is initiated by various extracellular and intracellular stressors, including viral infection (Guo et al., 2018), inflammation (Pasparakis and Vandenabeele, 2015), and factors detected by specific death receptors (e.g. FAS, TNFR1) or pathogen recognition receptors (Galluzzi et al., 2018; Frank and Vince, 2019) (Fig. 2). Importantly, these death receptors can also activate the extrinsic apoptosis pathway (Grootjans et al., 2017; Frank and Vince, 2019). At the molecular level, necroptosis critically depends on activation of the receptor-interacting serine/threonine-protein kinase (RIPK) 1/3 necrosome and mixed lineage kinase domain-like pseudokinase (MLKL), in the absence of caspase-8 activation, to ultimately cause cell membrane rupture (Galluzzi et al., 2018). Indeed, necroptosis is generally observed as a fall-back regulated cell death mechanism that is triggered when apoptosis is hindered, such as during pathogen infection (Brault and Oberst, 2017; Naderer and Fulcher, 2018).
Pyroptosis
Exogenous insults also extend to infection, and regulated cell death is proposed to contribute to immune defence against infections (Jorgensen et al., 2017). Pyroptosis is mediated by the cleavage of gasdermins, caspases (namely caspase-1), or granzymes; leading to the formation of pores in the cell membrane, lysis of the cell, and the release of inflammatory molecules (Frank and Vince, 2019) (Fig. 2). This type of regulated cell death is primarily observed in inflammatory cells, such as macrophages, and occurs most frequently upon infection with intracellular pathogens (Xia et al., 2019). As such, it is likely to form part of the host response to control bacterial, viral, fungal, or protozoan pathogens (Xia et al., 2019). While the sterile inflammatory response is required for organ development and tissue repair, dysregulation of this process may lead to inflammatory disease, for example asthma, Type 2 diabetes, and inflammatory liver diseases (Rock et al., 2010). Indeed, there is accumulating evidence that pyroptosis and inflammasome dysregulation may contribute to sterile inflammatory diseases, gynaecological diseases, autoimmune diseases, neuronal diseases, and even cancer (Li et al., 2021a; Yu et al., 2021). Moreover, cytokine dysregulation resulting in a pre-inflammatory phenotype that occurs with age, known as ‘inflammageing’ (Rea et al., 2018), has also been associated with pyroptosis (Mejias et al., 2018).
Parthanatos
Parthanatos is a poly[ADP-ribose] polymerase 1 (PARP1)-dependent and apoptosis-inducing factor (AIF)-mediated, caspase-independent cell death pathway, which is distinct from apoptosis, necroptosis, or other known forms of regulated cell death (Fig. 2). Parthanatos is associated with various diseases including several retinal diseases, Parkinson’s disease, stroke, heart attack, and diabetes (David et al., 2009). Parthanatos is triggered by an excessive ROS response, which leads to an accumulation of poly[ADP-ribose] (PAR) polymer and translocation of AIF from mitochondria to the nucleus, resulting in chromatin condensation and nuclear fragmentation (David et al., 2009; Wang et al., 2011). Parthanatos does have shared characteristics with necroptosis, including loss of membrane integrity and depletion of cellular energy stores (NAD and ATP) (Yu et al., 2003). However, cells undergoing parthanatos experience regulated chromatolysis without swelling and rupturing of cell membranes (Andrabi et al., 2008), as occurs during necroptosis. Key morphological features of parthanatos include shrunken, condensed nuclei, and membrane disintegration (Andrabi et al., 2008).
Timing and pathways of regulated cell death in ovarian development and function
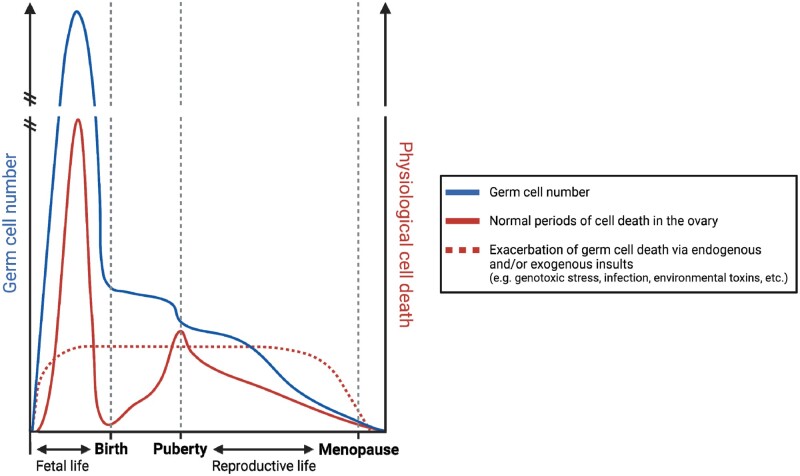
Regulation of cell death pathways is critical to orchestrating numerous aspects of ovarian development and function across the lifespan (Fig. 1). More than 99% of mammalian follicles will not reach ovulation, instead undergoing atresia (Wallace and Kelsey, 2010; Pelosi et al., 2015). It is well-established that follicle atresia is predominately mediated by apoptosis. In women, germ cell number peaks at ∼5 months gestation, with ∼6.8 million germ cells present in the ovary. This number falls to ∼1 million at birth, and by the onset of puberty, the follicle pool contains only 300 000 follicles (Baker, 1963). Despite this, over a woman’s reproductive life, only ∼400 oocytes will survive and undergo maturation to ovulation (Morita and Tilly, 1999). At ∼50 years of age, ovarian senescence and menopause is triggered when a critical threshold of <1000 follicles remain in the ovary (Faddy et al., 1992).
Whilst there is continuous loss of germ cells throughout life, there are two distinct waves during which large numbers of oocytes and primordial follicles are lost in a short period of time (Fig. 3). The first occurs prior to birth in humans, during which primordial follicle formation is occurring, and a second occurs at the onset of puberty. It is now widely accepted that granulosa cell apoptosis is primarily responsible for the atresia of growing follicles, whereas primordial follicle atresia is largely initiated by oocyte apoptosis (Vaskivuo and Tapanainen, 2003; Meng et al., 2018; Regan et al., 2018). However, alternative regulated cell death pathways can also trigger follicle atresia across the lifespan, especially following exposure to certain environmental stressors and toxicants.
Figure 3.
Timing of regulated cell death across the lifespan within the ovary in women. It is well-defined that multiple windows of increased germ cell loss across the female lifespan. These include immediately prior to birth and puberty. After puberty, there is a consistent decline in germ cell number across reproductive life, until menopause ensues. However, spikes in loss can be induced in response to endogenous and/or exogenous insults. Figure created using BioRender.
Ovarian reserve establishment
Germ cell loss occurs throughout the process of primordial follicle formation, predominately via apoptosis (McClellan et al., 2003). However, our analysis of studies performed in mice reveals some discrepancies in the exact timing of apoptosis. One of the earliest characterizations was by Bakken and McClanahan (1978), who identified germ cell degeneration (death) by their condensed nuclei containing rounded clumps of densely stained chromatin. This study reported increased germ cell degeneration during the last mitotic divisions, as oocytes become arrested in the first meiotic prophase (Bakken and McClanahan, 1978). At this time, germ cell nests begin to break down and facilitate primordial follicle assembly, culminating in loss of up to 98% of germ cells (Pepling and Spradling, 2001). Data examining germ cell loss during the early stages of meiosis (embryonic day 13.5–17.5 in mice; approximately mid-gestation in humans) are variable. Some reports highlight little to no germ cell loss and few TUNEL-positive-stained germ cells at this time (Pepling and Spradling, 2001). Meanwhile, others have identified a continuous decline and increased proportion of apoptotic germ cells during the first meiotic prophase compared to oogonia undergoing mitosis (Coucouvanis et al., 1993; McClellan et al., 2003). Thus, it is still debated by the field as to whether there are specific windows of germ cell loss during foetal development, or if this loss occurs continuously in the lead up to nest breakdown and follicle formation. Irrespective of the timing of loss, it is well-established that the key anti-apoptotic proteins (e.g. BCL-XL and BCL-2) and pro-apoptotic proteins (e.g. BAX, BMF, and PUMA) are clearly required for this process (Rucker et al., 2000; Flaws et al., 2006; Ke et al., 2013; Myers et al., 2014; Vaithiyanathan et al., 2016).
Extensive germ cell loss occurs during nest breakdown. It has been proposed that these dying cells could be acting as nurse-like cells that transfer important organelles (including centrioles, Golgi, and mitochondria) and the cytoplasm to a select few oogonia, before undergoing regulated cell death (Lei and Spradling, 2016). The remaining oogonia that acquired these additional cellular components become progressively larger, mature, and form the primordial follicle pool. Apoptosis is fundamental to orchestrating nest breakdown and primordial follicle assembly (Lei and Spradling, 2016). However, autophagy likely also plays a key role in this process (recently reviewed by Bhardwaj et al., 2022). Interestingly, inhibiting autophagy mediators using 3-methyladenine delays follicle formation and results in an increased number of cyst oocytes (Rodrigues et al., 2009; Zhihan et al., 2019). In support, germ cell survival immediately prior to and during follicle formation is severely impaired in mice with genetic loss of autophagy-related genes Atg7 and Becn1 (Gawriluk et al., 2011; Song et al., 2015). Lysosome amplification—a hallmark of autophagy—is also observed in oocytes upon birth and is most apparent in primordial follicle oocytes (Rodrigues et al., 2009). Lysine-specific demethylase 1 (LSD1)—a critical repressor of autophagy—is highly expressed in mouse foetal ovaries, but sharply reduces during the period of follicle assembly. Expression data, together with functional studies in vitro, indicate that LSD1 is an indispensable regulator of oocyte death during ovarian reserve establishment, via regulation of autophagy (He et al., 2020). During the foetal-to-neonatal transition in mammals, which is associated with a transformation in nutrient supply from the maternal–foetal blood interface to lactation, there is a disruption in nutrient supply to the ovary (Kuma et al., 2004). Interestingly, autophagy has been shown to play a critical role in nutrient stress adaptation to prevent excessive germ cell loss during this period in mice (Song et al., 2015; Sun et al., 2018). Together, these studies imply key roles for autophagy mediators in regulating oocyte survival, particularly during nest breakdown and primordial follicle formation.
Once established, primordial follicles enter meiotic arrest. Deemed ‘non-growing follicles’, the oocytes remain arrested at the diplotene stage of meiotic prophase 1, and the surrounding granulosa cells have low mitotic potential (Hartshorne et al., 2009). These primordial follicles represent the stockpile of oocytes available to females for their reproductive life. As such, these primordial follicle oocytes are some of the longest living cells in the mammalian body, and may remain arrested for decades in humans (Pelosi et al., 2015). These follicles will ultimately leave this period of dormancy, either by activation to continue through stepwise maturation via follicle development, or to undergo follicle atresia and oocyte loss. Regulation of this process and a balance between growth, survival, and atretic factors are essential in maintenance of normal reproductive function.
Puberty
During puberty, rising gonadotrophin levels cause dynamic physiological changes in the ovary, including the development of antral follicles to the Graafian (pre-ovulatory) stage and the onset of ovulation. Strikingly, this window also coincides with a significant spike in the loss of the ovarian reserve of primordial follicles, by approximately half during the adolescent/young adult period (ages 13–25 years) in humans (Wallace and Kelsey, 2010) and roughly two-thirds in mice (Allan et al., 2006; Bristol-Gould et al., 2006). This loss is thought to be gonadotrophin-mediated, though the precise mechanisms remain unknown (Liew et al., 2017). Notably, overexpression of LH in juvenile mice has been shown to trigger depletion of the primordial follicle reserve (Flaws et al., 1997). Conversely, LH and FSH suppression prevented follicle loss in a more recent study (Liew et al., 2017). Importantly, this primordial follicle loss is regulated by apoptosis, as demonstrated by the essential requirement for the pro-apoptotic BH3-only protein, BMF, in this process (Liew et al., 2017).
It remains unclear why such vast numbers of primordial follicles are eliminated at the time of sexual maturation. One explanation for this may be the fact that there are two separate populations of primordial follicles in the ovary that each have distinct functional roles. Class 1 primordial follicles, which are localized to the ovarian medulla, will activate and grow during pre-pubertal life but never be ovulated. These are fast growing, taking 19–21 days to reach maturity in mice (Gilchrist et al., 2001). They will contribute to the first wave of follicle activation, which is in turn likely required for the establishment of the hypothalamic–pituitary–ovarian axis, and puberty onset. Class 2 primordial follicles, located at the ovarian cortex, are slower growing (∼47 days) and are thought to represent the source of all mature ovulatory oocytes for fertilization (Mork et al., 2012; Zheng et al., 2014). Therefore, it is possible that the spike in oocyte loss observed at the transition of puberty involves the clearance of any remaining Class 1 primordial follicles in the medulla. While the two classes of follicles have not been clearly identified in human ovaries, the growth pattern of the first wave of activated follicles during foetal development is conserved in humans (Lintern-Moore et al., 1974; Peters et al., 1975). Moreover, in human ovaries, nearly 20%, 5%, and 0% of the primordial follicles in pre-pubertal, pubertal, and adult ovaries, respectively, are classed as morphologically abnormal (Anderson et al., 2014). Cultures of human cortical tissue containing primordial follicles and isolated pre-antral follicles from pre-pubertal and pubertal girls exhibited low activation rates and compromized oocyte growth respectively, compared to adult samples (Anderson et al., 2014). Thus, it can be speculated that these abnormal primordial follicles are either eliminated or preferentially activated before puberty onset and the commencement of ovulation in humans (Anderson et al., 2014). This provides an intriguing parallel to the two distinct types of primordial follicles identified by Mork et al. (2012) in mice (Anderson et al., 2014).
Follicle atresia throughout reproductive life
Atresia is a complex process that naturally occurs to regulate the follicle pool across the lifespan (Regan et al., 2018). It affects all stages of follicular development and involves multiple forms of regulated cell death. The highest incidence of follicular degeneration is observed when follicles become dependent on FSH, at the early antral follicle stage (Chun et al., 1996). Atresia is essential for maintaining ovarian homeostasis, and the dysregulation of atresia contributes to reproductive disorders, including PCOS and POI (Duncan, 2014).
Apoptosis
The regulation of intrinsic apoptosis ensures the maintenance of the number and the quality of the long-lived primordial follicle pool. This pathway is responsible for eliminating defective oocytes, which is paramount to sustain fertility and generate healthy offspring. Genotoxic stress (i.e. DNA damage) within oocytes—particularly in the form of double-stranded breaks—is extremely harmful to chromosome structure and overall DNA integrity. DNA damage can readily accumulate within primordial follicle oocytes as a consequence of normal cellular metabolism and increased levels of oxidative stress during the ageing process (Titus et al., 2013; Stringer et al., 2018; Winship et al., 2018). Additionally, DNA damage can be induced exogenously following exposure to various exogenous insults, including ionizing radiation, environmental toxicants, and certain chemotherapies causing extensive primordial oocyte apoptosis (Oktem and Oktay, 2007; Tatone et al., 2008; Soleimani et al., 2011; Winship et al., 2018).
In oocytes, intrinsic apoptosis is predominately regulated by TAp63α—an isoform of p63, which is the major p53 family member present. TAp63α, and its downstream effector PUMA, are primarily responsible for initiating oocyte apoptosis in response to genotoxic stress in vivo (Suh et al., 2006; Livera et al., 2008; Kerr et al., 2012b). Building on this work, a recent report showed that the ovaries in neonatal mice with a Trp63 exon 13 deletion (which leads to selective silencing of the TAp63α, but not the β isoform) were almost completely devoid of oocytes (Lena et al., 2021). This phenotype was a consequence of increased transcription of Puma and Noxa expression cause by the constitutively active TAp63β isoform. These data reveal that control of p63 signalling, and the intrinsic apoptosis pathway, is fundamentally important for oocyte maintenance.
Antral follicular degeneration is predominantly initiated by granulosa cell apoptosis. Activation of the death ligand–receptor system is the most common trigger of granulosa cell apoptosis, via the extrinsic apoptosis pathway specifically (Inoue et al., 2011; Chu et al., 2018). The FAS–FAS ligand (FAS-L) system has been localized to the human and rodent ovary, primarily in the granulosa and theca cells of unhealthy pre-antral and antral follicles, and in luteal cells of the corpus luteum (Albamonte et al., 2019), indicating a role in ovarian follicular atresia and luteolysis. Indeed, co-culture of interferon-γ pre-treated granulosa cells and denuded oocytes resulted in granulosa cell apoptosis, which could be blocked by an inhibitor of the FAS–FAS-L interaction (Hakuno et al., 1996). Additionally, new evidence suggests that the extrinsic apoptosis pathway may also regulate atresia of primordial follicles. A recent study examining the impact of checkpoint inhibitor immunotherapy on ovarian function in a mouse model revealed that TNF-α can directly induce primordial follicle loss via BID, which is a key member of the extrinsic apoptosis pathway (Winship et al., 2022).
Autophagy and autophagic cell death
Emerging evidence suggests that autophagic cell death plays a role in follicle atresia (recently reviewed by Bhardwaj et al., 2022). Granulosa cell apoptosis may also be triggered by the accumulation of autophagic vacuoles (autophagosomes) leading to the down-regulation of BCL-2 expression (Choi et al., 2010, 2011), suggesting that autophagy is closely related to apoptosis induction in granulosa cells. Interestingly, recent investigations show that different regulated cell death pathways beyond apoptosis play active roles in mediating follicle atresia, depending on the stage of follicle development. Pre-antral follicle atresia occurs largely via enhanced granulosa cell autophagy, meanwhile antral follicle atresia arises due to granulosa cell apoptosis (Meng et al., 2018). The oocyte residing within the atretic follicle may then be eliminated by mechanisms involving mediators common to both apoptosis and autophagy pathways (Escobar et al., 2008; Sanchez et al., 2012; Escobar et al., 2019). Unlike standard morphological features of apoptosis, oocytes within atretic follicles do not display normal chromatin compaction; however, they do display DNA fragmentation. Therefore, oocyte death may begin with autophagic degradation of cytoplasmic components, including mitochondria, which activates caspases that lead to DNA fragmentation without compaction; thus, triggering a non-conventional route of cell death.
There is, in fact, evidence that autophagy contributes to the regulation of oocyte death and follicle atresia at all stages of development, though its role in cell survival versus cell death appears to be complex. The expression of autophagy-related genes (e.g. those encoding ATG proteins, microtubule-associated proteins 1A/1B light chain 3 A and B [LC3A/B], beclin-1 [BECN1] and [LAMPs]) have been detected in follicles at all stages of development in rodents and pigs (Rodrigues et al., 2009; Choi et al., 2010; Gawriluk et al., 2011; Hale et al., 2017; Ullah et al., 2019; Leopardo et al., 2020). In mice, the relative mRNA expression of Becn1 is highest in primordial follicle oocytes compared with oocytes from primary, pre-antral, small antral, and large antral follicles, and protein is present in follicles (theca and granulosa cells) and oocytes of all stages including atretic follicles, but is absent from ovary epithelium (Gawriluk et al., 2011). In the rat, the expression pattern of LC3A appears to be restricted to granulosa cells with weak staining in thecal cells, but no staining in oocytes (Choi et al., 2010). LC3B protein is localized to the cytoplasm of oocytes and granulosa cells of all follicle stages as well as in steroidogenic cells of the corpus luteum (Ullah et al., 2019; Leopardo et al., 2020). Importantly, LC3B levels are significantly higher in ovaries of heat-stressed mice (Ullah et al., 2019) and after exposure to FSH (Zhou et al., 2017; Tang et al., 2021a). Less is known about the expression of these genes in humans; however, BECN1 and LC3A are present in the KGN immortalized human granulosa cell line (Yefimova et al., 2020; Li et al., 2021b). Importantly, a recent in vitro study demonstrated that human granulosa cells can remove apoptotic oocytes by unconventional autophagy-assisted phagocytosis (Yefimova et al., 2020), which may explain the expression of autophagy degradation machinery in these cells. LC3A is also expressed in cumulus cells from human cumulus–oocyte completes (COCs), with higher expression in cumulus cells classed as dysmature (Kang et al., 2018). Interestingly, oocytes from COCs with dysmature cumulus cells had a much lower fertilization rate, highlighting LC3A as a possible biomarker for lower quality human cumulus cells (Kang et al., 2018).
The involvement of autophagy, with or without cell death, has been reported in the establishment and maintenance of the primordial follicle reserve (Rodrigues et al., 2009; Gawriluk et al., 2011; Zhihan et al., 2019), follicle development and atresia (Meng et al., 2018), luteinization of granulosa cells and formation of corpus luteum (Tang et al., 2021b), and corpus luteum regression (Choi et al., 2011). Expression or activation of autophagy-related proteins is most evident in high stress conditions (such as starvation, heat, and hypoxia) and can suppress apoptotic signalling in the oocyte and/or granulosa cells in the ovary, promoting the survival of oocytes and follicles, and thereby ensuring fertility (Gannon et al., 2012; Hale et al., 2017; Watanabe and Kimura, 2018). Consistent with the concept that autophagy plays important survival roles in the ovary, deletion of the autophagy induction gene Atg7 in female mice leads to excessive germ cell loss during follicle formation, reduced ovarian reserve, and subfertility (Song et al., 2015). It has been proposed that autophagy protects immature oocytes from elimination by apoptosis, under starvation conditions (Song et al., 2015; Wang et al., 2017). Moreover, loss of function mutations in ATG7 and ATG9A are associated with autophagy impairment and ovarian failure in women (Delcour et al., 2019). On the other hand, elevated expression of autophagy-related genes within granulosa cells appears to be important for the normal and insult-induced atresia of antral and pre-ovulatory follicles (Shen et al., 2017, 2018; Gioia et al., 2019; Ma et al., 2019; Bhardwaj et al., 2022), but excessive or unregulated autophagy may be pathogenic. For example, increased autophagy has been observed in ovaries from women with PCOS (Li et al., 2018; Kumariya et al., 2021; Xie et al., 2021), which may indicate an important role for autophagy in ovarian homeostasis.
As a final note, in mature oocytes, BECN1—a key regulator of autophagosome formation and membrane trafficking—may also regulate chromosome segregation and cytokinesis during the last stages of meiosis, independent of its role in the autophagy pathway (You et al., 2016). Thus, it is important to consider that expression of proteins associated with autophagy does not necessarily imply autophagy is occurring.
Oxidative stress, necroptosis, and pyroptosis
ROS contribute to the physiological functions of follicles and human granulosa cells (Saller et al., 2012). Indeed, ROS are needed for various processes within the ovary, including ovulation (Shkolnik et al., 2011; Wang et al., 2022). However, accumulation of ROS from physiological stress (i.e. release of cortisol) or exposure to environmental and/or endogenous toxins can trigger various regulated cell death pathways and follicle atresia. Besides apoptosis and autophagy, necroptosis of granulosa cells and oocytes has been reported in the ovary in response to ROS accumulation. In studies performed in vitro, serum starving human granulosa cells causes generation of ROS and induces both necroptosis and apoptosis (Tsui et al., 2017). Indeed, mediators of necroptosis, such as phosphorylated MLKL, RIPK1, and RIPK3 proteins, are readily detected in the granulosa cells of pre-antral and antral follicles in macaque ovaries and human corpora lutea (Blohberger et al., 2015; Du et al., 2018). Moreover, a recent publication identified elevated gene expression of RIPK1 and RIPK3 in atretic, but not healthy, bovine follicles (McEvoy et al., 2021). Collectively, these data suggest that necroptosis, in concert with apoptosis and autophagy, may play a role in regulating late-stage follicle atresia, but limited mechanistic information is available.
In the ovary, granulosa cells of antral follicles are producers and targets of acetylcholine (ACh) (Mayerhofer et al., 2006). ACh is an important neurotransmitter that has been implicated in the regulation of cell viability, proliferation, gap junctional communication and intracellular calcium levels, as well as expression of transcription factors (Fritz et al., 2001, 2002; Kunz et al., 2002; Traut et al., 2009). Two esterases cleave and inactivate ACh—butyrylcholinesterase and acetylcholinesterase (AChE), with several splice variants of AChE (e.g. AChE-E, -S, and -R)—which results in isoforms that differ in subcellular localization and enzyme activity (Meshorer and Soreq, 2006). Importantly, the expression of splice variant AChE-R increases in response to oxidative stress (Härtl et al., 2011; Zimmermann, 2013) and circulating levels also increase with age in humans (Sklan et al., 2004). AChE-R can induce RIPK1-/MLKL-dependent necroptosis of granulosa cells (Blohberger et al., 2015), which can be blocked using key inhibitors of necroptosis (e.g. the RIPK1 inhibitor necrostatin-1 and MLKL-blocker necrosulfonamide) (Blohberger et al., 2015; Du et al., 2018). Locally inhibiting AChE using Huperzine A, via intrabursal injection, in vivo for 4 weeks in rats significantly increased the number of pre-antral follicles, corpora lutea, and pup numbers, highlighting an important role for ACh in follicular development and ovulation (Urra et al., 2016). Blocking the breakdown of ACh by inhibiting AChE (using Huperzine A) or interfering with necroptosis (necrostatin-1) did not improve follicle survival, but did promote oocyte development and growth of macaque follicles from the pre-antral to the small antral stage, and increased follicle granulosa cell number in vitro (Du et al., 2018). These studies strongly implicate granulosa cell necroptosis as an additional regulated cell death pathway that can be utilized during follicle atresia. However, the mechanism by which AChE-R induces necroptosis in granulosa cells remains to be determined.
Interestingly, oxidative stress can also prime the NLR family pyrin domain containing 3 (NLRP3) inflammasome (Bauernfeind et al., 2011; Won et al., 2015), resulting in caspase-1 activation and pyroptosis (Strowig et al., 2012). A recent study showed that polystyrene microplastics, which are transported to the ovary and taken in by the granulosa cells, result in the induction of NLRP3/caspase-1 signalling and pyroptosis activation in the ovary (Hou et al., 2021). In addition, a recent study in dairy cows demonstrated that culturing granulosa cells in the presence of non-esterified fatty acids (NEFAs) induces oxidative stress, pyroptosis, and inflammation (Wang et al., 2020). Specifically, NEFAs activate the toll-like receptor 4 (TLR4)/NF-κB pathway, increase the production of NLRP3 and caspase-1, and trigger granulosa cells to release inflammatory cytokines interleukin (IL)-1β and IL-6. Importantly, these effects were reversed when the granulosa cells were pre-treated with antioxidant N-acetylcysteine, validating the role of oxidative stress during NEFA-induced pyroptosis. The involvement of NEFAs in this process is particularly interesting as high levels of NEFAs are a hallmark of various metabolic diseases, including obesity, Type 2 diabetes, and ketosis in humans and animals (Baddela et al., 2020). These circulating NEFAs can enter the ovary and the follicular fluid, and negatively impact on the steroidogenic functions of granulosa cells and oocyte quality (Yang et al., 2012; Valckx et al., 2014; Calonge et al., 2018).
Ovarian ageing
Reproductive ageing coincides with a decline in ovarian follicle number, leading to loss of fertility and endocrine function, and eventually menopause. Notably, emerging evidence indicates that the ovary naturally transitions to a low-level inflammatory microenvironment with advancing maternal age, termed ‘inflammageing’ (Lliberos et al., 2021; Camaioni et al., 2022; Umehara et al., 2022). The NLRP3/apoptosis-associated speck-like protein (ASC) inflammasome, which activates caspase-1, appears to be central to this process, raising the possibility that pyroptosis might contribute to age-associated follicle loss. Although pyroptosis has not been directly studied during ovarian ageing, this hypothesis is supported by recent reports demonstrating that genetic loss or pharmacological inhibition of NLRP3 or ASC reduces caspase-1 levels in the ovary, increases oocyte number, and delays ovarian ageing (Lliberos et al., 2020; Navarro-Pando et al., 2021). Furthermore, the granulosa cells of women with POI exhibit elevated NLRP3, caspase-1, and IL-1α levels (Navarro-Pando et al., 2021). Further investigations of how pyroptosis is regulated in the ovary will provide valuable therapeutic targets to potentially delay natural ovarian ageing.
Some new evidence suggests that parthanatos may also contribute to premature ovarian ageing. Cumulus granulosa cells collected from women with diminished ovarian reserve showed increased levels of nuclear purified PAR and AIF (Batnasan et al., 2020), suggesting a role for PARP-dependent cell death in diminished ovarian reserve pathophysiology. Therefore, inhibition of parthanatos, amongst other regulated cell death pathways, may prove useful for patients with diminished ovarian reserve.
Future directions
Distinguishing between oocyte versus somatic cell death in ovarian follicles
Granulosa cells are specialized somatic cells in the ovary, vital for oocyte survival and female fertility. When follicles are formed, oocytes not surrounded by granulosa cells are eliminated. Impaired granulosa cell function also dysregulates oocyte growth and causes POI, characterized by early loss of fertility and hormone production (Uda et al., 2004). Recent endeavours to derive human or mouse oocytes from induced pluripotent stem cells rely exclusively on mouse granulosa cells to support the germ cell-like cells (Sarma et al., 2019; Stringer and Western, 2019). Collectively, these observations reveal that granulosa cells have unique and essential functional properties that cannot be replaced by other somatic cell types.
In response to activation signals, primordial follicles give rise to large, hormone-producing follicles, and mature ovulatory oocytes. The founding population of ∼5–8 granulosa cells present in a primordial follicle undergoes clonal divisions to eventually produce >2000 granulosa cells that support a mature oocyte (Hirshfield, 1991). After primordial follicle formation, there is no evidence of new granulosa cell formation from other somatic cell types (Zhang et al., 2014). Thus, all mature granulosa cells appear to be clones of primordial follicle granulosa cells. As oocytes cannot survive without these essential granulosa cells, granulosa cell apoptosis invariably causes follicle atresia. However, the possibility that primordial follicle granulosa cells are susceptible to ageing and exogenous insults has not been investigated. Understanding the regulated cell death pathways utilized by each cell type comprising ovarian follicles is important to better understand the effects of maternal ageing, and to develop appropriate fertility preservation strategies for female cancer patients.
Role of the ovarian environment in regulated cell death of ovarian follicles
Studies of the ovarian environment, including the stroma and other supporting somatic cell types, have emerged as a recent area of interest (Kinnear et al., 2020). Some studies have established that fibrosis is one early hallmark of the aging ovarian stroma (Briley et al., 2016; Amargant et al., 2020; Umehara et al., 2022). It has thus been proposed that this altered microenvironment could contribute to the age-associated decline in oocyte number and/or quality. However, a direct link between the ovarian stroma and regulated cell death in ovarian follicles or other somatic cell types is lacking, and should be the focus of further investigation.
Targeting regulated cell death pathways to protect fertility
Inhibiting key mediators of oocyte death is a promising strategy for protecting primordial follicles from anti-cancer treatment and age-mediated depletion. Such strategies require characterization of the pathways and specific mediators involved in order to identify suitable targets. Of relevance to cancer treatment, Bax–/– mice have prolonged fertility after chemotherapy (Kujjo et al., 2010). Similarly, irradiated TAp63–/– (Stringer et al., 2020), Puma–/– (Kerr et al., 2012b), and checkpoint kinase 2 (Chk2)–/– mice (Bolcun-Filas et al., 2014; Rinaldi et al., 2017) remain fertile after treatment with radiation or chemotherapy, unlike their wild-type counterparts, demonstrating the potential of this strategy for fertility preservation. Translation of this information is currently limited by the availability of effective small-molecule inhibitors to these proteins. However, an inhibitor of CHK2 does exist, and its transient use reduced irradiation-mediated primordial follicle loss in mice, leading to the generation of healthy offspring (Rinaldi et al., 2017).
Targeting other regulated cell death pathways, independently or in concert with apoptosis, may also provide novel fertility preservation strategies. Indeed, investigations on the use of AChE and necroptosis inhibitors to improve folliculogenesis (Urra et al., 2016; Du et al., 2018), or inflammasome inhibitors to delay ovarian ageing (Navarro-Pando et al., 2021; Umehara et al., 2022), are exciting potential avenues for fertility preservation. However, more studies are needed to understand when and how these pathways are activated in the ovary, in order to determine the best timing and suitability of these therapies.
Conclusion
The contribution of apoptosis to the processes of follicle formation during foetal development and around birth, as well as throughout other life stages, is well defined. However, here, we highlight substantial gaps in knowledge of the localization, activation, and contribution of key players from other types of regulated cell death pathways in ovarian follicles. Understanding the relative importance of all the different regulated cell death pathways, and how they are activated in both granulosa cells and oocytes, is vital to identify other potential therapeutic targets for fertility preservation strategies.
Acknowledgements
This work was made possible through Victorian State Government Operational Infrastructure Support and Australian Government National Health and Medical Research council Independent Research Institutes Infrastructure Support Scheme. Figures were created using BioRender.
Contributor Information
Jessica M Stringer, Department of Anatomy and Developmental Biology, Monash Biomedicine Discovery Institute, Monash University, Clayton, VIC, Australia.
Lauren R Alesi, Department of Anatomy and Developmental Biology, Monash Biomedicine Discovery Institute, Monash University, Clayton, VIC, Australia.
Amy L Winship, Department of Anatomy and Developmental Biology, Monash Biomedicine Discovery Institute, Monash University, Clayton, VIC, Australia.
Karla J Hutt, Department of Anatomy and Developmental Biology, Monash Biomedicine Discovery Institute, Monash University, Clayton, VIC, Australia.
Data availability
No new data were generated or analysed in support of this research.
Authors’ roles
J.M.S. and K.J.H. conceived. L.R.A. designed tables and figures. All authors contributed to writing and editing the manuscript.
Funding
This work was supported by funding from the Australian Research Council; K.J.H.—FT190100265 and A.L.W.—DE21010037, and the National Health and Medical Research council; J.M.S.—2011299. L.R.A. is supported by an Australian Government Research Training Program scholarship and a Monash Graduate Excellence Scholarship.
Conflict of interest
The authors declare no competing financial, or other interests.
References
- Albamonte MI, Albamonte MS, Bou-Khair RM, Zuccardi L, Vitullo AD.. The ovarian germinal reserve and apoptosis-related proteins in the infant and adolescent human ovary. J Ovarian Res 2019;12:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan CM, Wang Y, Jimenez M, Marshan B, Spaliviero J, Illingworth P, Handelsman DJ.. Follicle-stimulating hormone increases primordial follicle reserve in mature female hypogonadal mice. J Endocrinol 2006;188:549–557. [DOI] [PubMed] [Google Scholar]
- Amargant F, Manuel SL, Tu Q, Parkes WS, Rivas F, Zhou LT, Rowley JE, Villanueva CE, Hornick JE, Shekhawat GS. et al. Ovarian stiffness increases with age in the mammalian ovary and depends on collagen and hyaluronan matrices. Aging Cell 2020;19:e13259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RA, McLaughlin M, Wallace WHB, Albertini DF, Telfer EE.. The immature human ovary shows loss of abnormal follicles and increasing follicle developmental competence through childhood and adolescence. Hum Reprod 2014;29:97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrabi SA, Dawson TM, Dawson VL.. Mitochondrial and nuclear cross talk in cell death: parthanatos. Ann N Y Acad Sci 2008;1147:233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubrey BJ, Kelly GL, Janic A, Herold MJ, Strasser A.. How does p53 induce apoptosis and how does this relate to p53-mediated tumour suppression? Cell Death Differ 2018;25:104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddela VS, Sharma A, Vanselow J.. Non-esterified fatty acids in the ovary: friends or foes? Reprod Biol Endocrinol 2020;18:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TG. A quantitative and cytological study of germ cells in human ovaries. Proc R Soc Lond B Biol Sci 1963;158:417–433. [DOI] [PubMed] [Google Scholar]
- Bakken AH, McClanahan M.. Patterns of RNA synthesis in early meiotic prophase oocytes from fetal mouse ovaries. Chromosoma 1978;67:21–40. [DOI] [PubMed] [Google Scholar]
- Batnasan E, Xie S, Zhang Q, Li Y.. Observation of parthanatos involvement in diminished ovarian reserve patients and melatonin’s protective function through inhibiting ADP-ribose (PAR) expression and preventing AIF translocation into the nucleus. Reprod Sci 2020;27:75–86. [DOI] [PubMed] [Google Scholar]
- Bauernfeind F, Bartok E, Rieger A, Franchi L, Núñez G, Hornung V.. Cutting edge: reactive oxygen species inhibitors block priming, but not activation, of the NLRP3 inflammasome. J Immunol 2011;187:613–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkel C, Cacan E.. Differential expression and copy number variation of gasdermin (GSDM) family members, pore-forming proteins in pyroptosis, in normal and malignant serous ovarian tissue. Inflammation 2021;44:2203–2216. [DOI] [PubMed] [Google Scholar]
- Bhardwaj JK, Paliwal A, Saraf P, Sachdeva SN.. Role of autophagy in follicular development and maintenance of primordial follicular pool in the ovary. J Cell Physiol 2022;237:1157–1170. [DOI] [PubMed] [Google Scholar]
- Bialik S, Dasari SK, Kimchi A.. Autophagy-dependent cell death – where, how and why a cell eats itself to death. J Cell Sci 2018;131:jcs215152. [DOI] [PubMed] [Google Scholar]
- Blohberger J, Kunz L, Einwang D, Berg U, Berg D, Ojeda SR, Dissen GA, Frohlich T, Arnold GJ, Soreq H. et al. Readthrough acetylcholinesterase (AChe-R) and regulated necrosis: pharmacological targets for the regulation of ovarian functions? Cell Death Dis 2015;6:e1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolcun-Filas E, Rinaldi VD, White ME, Schimenti JC.. Reversal of female infertility by CHK2 ablation reveals the oocyte DNA damage checkpoint pathway. Science 2014;343:533–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brault M, Oberst A.. Controlled detonation: evolution of necroptosis in pathogen defense. Immunol Cell Biol 2017;95:131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briley SM, Jasti S, McCracken JM, Hornick JE, Fegley B, Pritchard MT, Duncan FE.. Reproductive age-associated fibrosis in the stroma of the mammalian ovary. Reproduction 2016;152:245–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bristol-Gould SK, Kreeger PK, Selkirk CG, Kilen SM, Mayo KE, Shea LD, Woodruff TK.. Fate of the initial follicle pool: empirical and mathematical evidence supporting its sufficiency for adult fertility. Dev Biol 2006;298:149–154. [DOI] [PubMed] [Google Scholar]
- Cai H, Liu B, Wang H, Sun G, Feng L, Chen Z, Zhou J, Zhang J, Zhang T, He M. et al. SP1 governs primordial folliculogenesis by regulating pregranulosa cell development in mice. J Mol Cell Biol 2020;12:230–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calonge RN, Kireev R, Guijarro A, Cortes S, Andres C, Caballero P.. Lipid dysregulation in seminal and follicular fluids could be related with male and female infertility. Endocrinol Metab Int J 2018;6:00156. [Google Scholar]
- Camaioni A, Ucci MA, Campagnolo L, De Felici M, Klinger FG;. Italian Society of Embryology, Reproduction and Research (SIERR). The process of ovarian aging: it is not just about oocytes and granulosa cells. J Assist Reprod Genet 2022;39:783–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Li Y, Zhang Y, Wang X, Sun F, Liu Y.. Conditional deletion of Wntless in granulosa cells causes impaired corpora lutea formation and subfertility. Aging (Albany NY) 2020;13:1001–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Jo M, Lee E, Choi D.. Induction of apoptotic cell death via accumulation of autophagosomes in rat granulosa cells. Fertil Steril 2011;95:1482–1486. [DOI] [PubMed] [Google Scholar]
- Choi JY, Jo MW, Lee EY, Yoon BK, Choi DS.. The role of autophagy in follicular development and atresia in rat granulosa cells. Fertil Steril 2010;93:2532–2537. [DOI] [PubMed] [Google Scholar]
- Chu Y-L, Xu Y-R, Yang W-X, Sun Y.. The role of FSH and TGF-β superfamily in follicle atresia. Aging (Albany NY) 2018;10:305–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun S, Eisenhauer KM, Minami S, Billig H, Perlas E, Hsueh A.. Hormonal regulation of apoptosis in early antral follicles: follicle-stimulating hormone as a major survival factor. Endocrinology 1996;137:1447–1456. [DOI] [PubMed] [Google Scholar]
- Costa L, Moreia-Pinto B, Felgueira E, Ribeiro A, Rebelo I, Fonseca BM.. The major endocannabinoid anandamide (AEA) induces apoptosis of human granulosa cells. Prostaglandins Leukot Essent Fatty Acids 2021;171:102311. [DOI] [PubMed] [Google Scholar]
- Coucouvanis EC, Sherwood SW, Carswell-Crumpton C, Spack EG, Jones PP.. Evidence that the mechanism of prenatal germ cell death in the mouse is apoptosis. Exp Cell Res 1993;209:238–247. [DOI] [PubMed] [Google Scholar]
- Cuervo AM. Autophagy: in sickness and in health. Trends Cell Biol 2004;14:70–77. [DOI] [PubMed] [Google Scholar]
- Cui L, Sheng Y, Sun M, Hu J, Qin Y, Chen Z-J.. Chronic pelvic inflammation diminished ovarian reserve as indicated by serum anti-Müllerian hormone. PLoS One 2016;11:e0156130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David KK, Andrabi SA, Dawson TM, Dawson VL.. Parthanatos, a messenger of death. Front Biosci (Landmark Ed) 2009;14:1116–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delcour C, Amazit L, Patino LC, Magnin F, Fagart J, Delemer B, Young J, Laissue P, Binart N, Beau I.. ATG7 and ATG9A loss-of-function variants trigger autophagy impairment and ovarian failure. Genet Med 2019;21:930–938. [DOI] [PubMed] [Google Scholar]
- Dello Stritto MR, Bauer B, Barraud P, Jantsch V.. DNA topoisomerase 3 is required for efficient germ cell quality control. J Cell Biol 2021;220:e202012057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton D, Kumar S.. Autophagy-dependent cell death. Cell Death Differ 2019;26:605–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Bagnjuk K, Lawson MS, Xu J, Mayerhofer A.. Acetylcholine and necroptosis are players in follicular development in primates. Sci Rep 2018;8:6166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan WC. A guide to understanding polycystic ovary syndrome (PCOS). J Fam Plann Reprod Health Care 2014;40:217–225. [DOI] [PubMed] [Google Scholar]
- Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol 2007;35:495–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar ML, Echeverría OM, Ortíz R, Vázquez-Nin GH.. Combined apoptosis and autophagy, the process that eliminates the oocytes of atretic follicles in immature rats. Apoptosis 2008;13:1253–1266. [DOI] [PubMed] [Google Scholar]
- Escobar ML, Echeverria OM, Palacios-Martínez S, Juárez-Chavero S, Sánchez-Sánchez L, Vázquez-Nin GH.. Beclin 1 interacts with active caspase-3 and BAX in oocytes from atretic follicles in the rat ovary. J Histochem Cytochem 2019;67:873–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faddy MJ, Gosden RG, Gougeon A, Richardson SJ, Nelson JF.. Accelerated disappearance of ovarian follicles in mid-life: implications for forecasting menopause. Hum Reprod 1992;7:1342–1346. [DOI] [PubMed] [Google Scholar]
- Feng S, Wan S, Liu S, Wang W, Tang M, Bai L, Zhu Y.. LARS2 regulates apoptosis via ros-mediated mitochondrial dysfunction and endoplasmic reticulum stress in ovarian granulosa cells. Oxid Med Cell Longev 2022;2022:5501346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay JK, Hutt KJ, Hickey M, Ra A.. How is the number of primordial follicles in the ovarian reserve established? Biol Reprod 2015;93:111. [DOI] [PubMed] [Google Scholar]
- Flaws J, Marion S, Miller K, Christian P, Babus J, Hoyer P.. Effect of BCL-2 overexpression in mice on ovotoxicity caused by 4-vinylcyclohexene. Toxicol Appl Pharmacol 2006;215:51–56. [DOI] [PubMed] [Google Scholar]
- Flaws JA, Abbud R, Mann RJ, Nilson JH, Hirshfield AN.. Chronically elevated luteinizing hormone depletes primordial follicles in the mouse ovary. Biol Reprod 1997;57:1233–1237. [DOI] [PubMed] [Google Scholar]
- Frank D, Vince JE.. Pyroptosis versus necroptosis: similarities, differences, and crosstalk. Cell Death Differ 2019;26:99–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz S, Kunz L, Dimitrijevic N, Grünert R, Heiss C, Mayerhofer A.. Muscarinic receptors in human luteinized granulosa cells: activation blocks gap junctions and induces the transcription factor early growth response factor-1. J Clin Endocrinol Metabol 2002;87:1362–1367. [DOI] [PubMed] [Google Scholar]
- Fritz S, Wessler I, Breitling R, Rossmanith W, Ojeda S, Dissen G, Amsterdam A, Mayerhofer A.. Expression of muscarinic receptor types in the primate ovary and evidence for nonneuronal acetylcholine synthesis. J Clin Endocrinol Metabol 2001;86:349–354. [DOI] [PubMed] [Google Scholar]
- Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews DW. et al. Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell Death Differ 2018;25:486–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon AM, Stampfli MR, Foster WG.. Cigarette smoke exposure leads to follicle loss via an alternative ovarian cell death pathway in a mouse model. Toxicol Sci 2012;125:274–284. [DOI] [PubMed] [Google Scholar]
- Gawriluk TR, Hale AN, Flaws JA, Dillon CP, Green DR, Rucker EB 3rd. Autophagy is a cell survival program for female germ cells in the murine ovary. Reproduction 2011;141:759–765. [DOI] [PubMed] [Google Scholar]
- Gilchrist RB, Ritter LJ, Armstrong DT.. Mouse oocyte mitogenic activity is developmentally coordinated throughout folliculogenesis and meiotic maturation. Dev Biol 2001;240:289–298. [DOI] [PubMed] [Google Scholar]
- Gioia L, Festuccia C, Colapietro A, Gloria A, Contri A, Valbonetti L.. Abundances of autophagy-related protein LC3B in granulosa cells, cumulus cells, and oocytes during atresia of pig antral follicles. Anim Reprod Sci 2019;211:106225. [DOI] [PubMed] [Google Scholar]
- Goldschneider D, Mehlen P.. Dependence receptors: a new paradigm in cell signaling and cancer therapy. Oncogene 2010;29:1865–1882. [DOI] [PubMed] [Google Scholar]
- Gonen N, Casper RF, Jurisicova A, Yung Y, Friedman-Gohas M, Orvieto R, Haas J.. Does gonadotropin-releasing hormone agonist cause luteolysis by inducing apoptosis of the human granulosa-luteal cells? J Assist Reprod Genet 2021;38:2301–2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grootjans S, Vanden Berghe T, Vandenabeele P.. Initiation and execution mechanisms of necroptosis: an overview. Cell Death Differ 2017;24:1184–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guicciardi ME, Gores GJ.. Life and death by death receptors. FASEB J 2009;23:1625–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Gilley RP, Fisher A, Lane R, Landsteiner VJ, Ragan KB, Dovey CM, Carette JE, Upton JW, Mocarski ES. et al. Species-independent contribution of ZBP1/DAI/DLM-1-triggered necroptosis in host defense against HSV1. Cell Death Dis 2018;9:816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakuno N, Koji T, Yano T, Kobayashi N, Tsutsumi O, Taketani Y, Nakane PK.. FAS/APO-1/CD95 system as a mediator of granulosa cell apoptosis in ovarian follicle atresia. Endocrinology 1996;137:1938–1948. [DOI] [PubMed] [Google Scholar]
- Hale BJ, Hager CL, Seibert JT, Selsby JT, Baumgard LH, Keating AF, Ross JW.. Heat stress induces autophagy in pig ovaries during follicular development. Biol Reprod 2017;97:426–437. [DOI] [PubMed] [Google Scholar]
- Härtl R, Gleinich A, Zimmermann M.. Dramatic increase in readthrough acetylcholinesterase in a cellular model of oxidative stress. J Neurochem 2011;116:1088–1096. [DOI] [PubMed] [Google Scholar]
- Hartshorne GM, Lyrakou S, Hamoda H, Oloto E, Ghafari F.. Oogenesis and cell death in human prenatal ovaries: what are the criteria for oocyte selection? Mol Hum Reprod 2009;15:805–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M, Zhang T, Zhu Z, Qin S, Wang H, Zhao L, Zhang X, Hu J, Wen J, Cai H.. LSD1 contributes to programmed oocyte death by regulating the transcription of autophagy adaptor SQSTM1/p62. Aging Cell 2020;19:e13102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshfield AN. Development of follicles in the mammalian ovary. Int Rev Cytol 1991;124:43–101. [DOI] [PubMed] [Google Scholar]
- Hou J, Lei Z, Cui L, Hou Y, Yang L, An R, Wang Q, Li S, Zhang H, Zhang L.. Polystyrene microplastics lead to pyroptosis and apoptosis of ovarian granulosa cells via NLRP3/caspase-1 signaling pathway in rats. Ecotoxicol Environ Saf 2021;212:112012. [DOI] [PubMed] [Google Scholar]
- Inoue N, Matsuda F, Goto Y, Manabe N.. Role of cell-death ligand-receptor system of granulosa cells in selective follicular atresia in porcine ovary. J Reprod Dev 2011;57:169–175. [DOI] [PubMed] [Google Scholar]
- Jorgensen I, Rayamajhi M, Miao EA.. Programmed cell death as a defence against infection. Nat Rev Immunol 2017;17:151–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang W, Ishida E, Yamatoya K, Nakamura A, Miyado M, Miyamoto Y, Iwai M, Tatsumi K, Saito T, Saito K. et al. Autophagy-disrupted LC3 abundance leads to death of supporting cells of human oocytes. Biochem Biophys Rep 2018;15:107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke F, Bouillet P, Kaufmann T, Strasser A, Kerr J, Voss AK.. Consequences of the combined loss of BOK and BAK or BOK and BAX. Cell Death Dis 2013;4:e650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JB, Brogan L, Myers M, Hutt KJ, Mladenovska T, Ricardo S, Hamza K, Scott CL, Strasser A, Findlay JK.. The primordial follicle reserve is not renewed after chemical or gamma-irradiation mediated depletion. Reproduction 2012a;143:469–476. [DOI] [PubMed] [Google Scholar]
- Kerr JB, Hutt KJ, Michalak EM, Cook M, Vandenberg CJ, Liew SH, Bouillet P, Mills A, Scott CL, Findlay JK. et al. DNA damage-induced primordial follicle oocyte apoptosis and loss of fertility require Tap63-mediated induction of PUMA and NOXA. Mol Cell 2012b;48:343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JB, Myers M, Anderson RA.. The dynamics of the primordial follicle reserve. Reproduction 2013;146:R205–R215. [DOI] [PubMed] [Google Scholar]
- Kinnear HM, Tomaszewski CE, Chang FL, Moravek MB, Xu M, Padmanabhan V, Shikanov A.. The ovarian stroma as a new frontier. Reproduction 2020;160:R25–R39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kist M, Vucic D.. Cell death pathways: intricate connections and disease implications. EMBO J 2021;40:e106700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriel J, Loos B.. The good, the bad and the autophagosome: exploring unanswered questions of autophagy-dependent cell death. Cell Death Differ 2019;26:640–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G, Levine B.. Autophagic cell death: the story of a misnomer. Nat Rev Mol Cell Biol 2008;9:1004–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujjo LL, Laine T, Pereira RJ, Kagawa W, Kurumizaka H, Yokoyama S, Perez GI.. Enhancing survival of mouse oocytes following chemotherapy or aging by targeting BAX and RAD51. PLoS One 2010;5:e9204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, Yoshimori T, Ohsumi Y, Tokuhisa T, Mizushima N.. The role of autophagy during the early neonatal starvation period. Nature 2004;432:1032–1036. [DOI] [PubMed] [Google Scholar]
- Kumariya S, Ubba V, Jha RK, Gayen JR.. Autophagy in ovary and polycystic ovary syndrome: role, dispute and future perspective. Autophagy 2021;17:2706–2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz L, Thalhammer A, Berg FD, Berg U, Duffy DM, Stouffer RL, Dissen GA, Ojeda SR, Mayerhofer A.. Ca2+-activated, large conductance K+ channel in the ovary: identification, characterization, and functional involvement in steroidogenesis. J Clin Endocrinol Metab 2002;87:5566–5574. [DOI] [PubMed] [Google Scholar]
- Lei L, Spradling AC.. Mouse oocytes differentiate through organelle enrichment from sister cyst germ cells. Science 2016;352:95–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lena AM, Rossi V, Osterburg S, Smirnov A, Osterburg C, Tuppi M, Cappello A, Amelio I, Dötsch V, Felici D. et al. The p63 c-terminus is essential for murine oocyte integrity. Nat Commun 2021;12:383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopardo NP, Velazquez ME, Cortasa S, Gonzalez CR, Vitullo AD.. A dual death/survival role of autophagy in the adult ovary of Lagostomus maximus (Mammalia–Rodentia). PLoS One 2020;15:e0232819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Meng X, Liu S, Li W, Zhang X, Zhou J, Yao W, Dong C, Liu Z, Zhou J. et al. Oocytes and hypoxanthine orchestrate the G2-M switch mechanism in ovarian granulosa cells. Development 2020;147:dev184838. [DOI] [PubMed] [Google Scholar]
- Li CJ, Lin LT, Tsai HW, Wen ZH, Tsui KH.. Phosphoglycerate mutase family member 5 maintains oocyte quality via mitochondrial dynamic rearrangement during aging. Aging Cell 2022;21:e13546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, You Y, Bi F-F, Zhang T-N, Jiao J, Wang T-R, Zhou Y-M, Shen Z-Q, Wang X-X, Yang Q.. Autophagy is activated in the ovarian tissue of polycystic ovary syndrome. Reproduction 2018;155:85–92. [DOI] [PubMed] [Google Scholar]
- Li J, Yuan J.. Caspases in apoptosis and beyond. Oncogene 2008;27:6194–6206. [DOI] [PubMed] [Google Scholar]
- Li M. The role of p53 up-regulated modulator of apoptosis (PUMA) in ovarian development, cardiovascular and neurodegenerative diseases. Apoptosis 2021;26:235–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Zheng G, Li B, Tang L.. Pyroptosis: a promising therapeutic target for noninfectious diseases. Cell Prolif 2021a;54:e13137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Liu Y-D, Zhou X-Y, Zhang J, Wu X-M, Yang Y-Z, Chen Y-X, Zhang X-F, Li X, Ma L-Z. et al. Let-7e modulates the proliferation and the autophagy of human granulosa cells by suppressing p21 signaling pathway in polycystic ovary syndrome without hyperandrogenism. Mol Cell Endocrinol 2021b;535:111392. [DOI] [PubMed] [Google Scholar]
- Liew SH, Nguyen Q-N, Strasser A, Findlay JK, Hutt KJ.. The ovarian reserve is depleted during puberty in a hormonally driven process dependent on the pro-apoptotic protein BMF. Cell Death Dis 2017;8:e2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew SH, Vaithiyanathan K, Hutt KJ.. Taking control of the female fertile lifespan: a key role for BCL-2 family proteins. Reprod Fertil Dev 2016;28:864–871. [DOI] [PubMed] [Google Scholar]
- Lintern-Moore S, Peters H, Moore GP, Faber M.. Follicular development in the infant human ovary. J Reprod Fertil 1974;39:53–64. [DOI] [PubMed] [Google Scholar]
- Liu C, Li S, Li Y, Tian J, Sun X, Song T, Yan G, Ding L, Sun H.. Growth hormone ameliorates the age-associated depletion of ovarian reserve and decline of oocyte quality via inhibiting the activation of Fos and Jun signaling. Aging 2021;13:6765–6781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livera G, Petre-Lazar B, Guerquin M-J, Trautmann E, Coffigny H, Habert R.. p63 null mutation protects mouse oocytes from radio-induced apoptosis. Reproduction 2008;135:3–12. [DOI] [PubMed] [Google Scholar]
- Lliberos C, Liew SH, Mansell A, Hutt KJ.. The inflammasome contributes to depletion of the ovarian reserve during aging in mice. Front Cell Dev Biol 2020;8:628473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lliberos C, Liew SH, Zareie P, Gruta NL, Mansell A, Hutt K.. Evaluation of inflammation and follicle depletion during ovarian ageing in mice. Sci Rep 2021;11:278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Ding F, Chen Y, Ke S, Yuan S, Qiu H, Xiao L, Yu Y.. Deficiency of C1QL1 reduced murine ovarian follicle reserve through intraovarian and endocrine control. Endocrinology 2022;163:bqac048. [DOI] [PubMed] [Google Scholar]
- Ma L, Zheng Y, Tang X, Gao H, Liu N, Gao Y, Hao L, Liu S, Jiang Z.. miR-21-3p inhibits autophagy of bovine granulosa cells by targeting VEGFA via PI3K/AKT signaling. Reproduction 2019;158:441–452. [DOI] [PubMed] [Google Scholar]
- Makowczenko KG, Jastrzebski JP, Paukszto L, Dobrzyn K, Kiezun M, Smolinska N, Kaminski T.. Chemerin impact on alternative mRNA transcription in the porcine luteal cells. Cells 2022;11:715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcozzi S, Rossi V, Salustri A, De Felici M, Klinger FG.. Programmed cell death in the human ovary. Minerva Ginecol 2018;70:549–560. [DOI] [PubMed] [Google Scholar]
- Mayerhofer A, Kunz L, Krieger A, Proskocil B, Spindel E, Amsterdam A, Dissen GA, Ojeda SR, Wessler I.. FSH regulates acetycholine production by ovarian granulosa cells. Reprod Biol Endocrinol 2006;4:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClellan KA, Gosden R, Taketo T.. Continuous loss of oocytes throughout meiotic prophase in the normal mouse ovary. Dev Biol 2003;258:334–348. [DOI] [PubMed] [Google Scholar]
- McEvoy MJ, Sinderewicz E, Creedon L, McAfee M, Jonczyk AW, Piotrowska-Tomala KK, Skarzynski DJ.. Death processes in bovine theca and granulosa cells modelled and analysed using a systems biology approach. Int J Mol Sci 2021;22:4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejias NH, Martinez CC, Stephens ME, de Rivero Vaccari JP.. Contribution of the inflammasome to inflammaging. J Inflamm (Lond) 2018;15:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng L, Jan SZ, Hamer G, van Pelt AM, van der Stelt I, Keijer J, Teerds KJ.. Preantral follicular atresia occurs mainly through autophagy, while antral follicles degenerate mostly through apoptosis. Biol Reprod 2018;99:853–863. [DOI] [PubMed] [Google Scholar]
- Meshorer E, Soreq H.. Virtues and woes of ache alternative splicing in stress-related neuropathologies. Trends Neurosci 2006;29:216–224. [DOI] [PubMed] [Google Scholar]
- Moldoveanu T, Czabotar PE.. BAX, BAK, and BOK: a coming of age for the BCL-2 family effector proteins. Cold Spring Harb Perspect Biol 2020;12:a036319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita Y, Tilly JL.. Oocyte apoptosis: like sand through an hourglass. Dev Biol 1999;213:1–17. [DOI] [PubMed] [Google Scholar]
- Mork L, Maatouk DM, McMahon JA, Guo JJ, Zhang P, McMahon AP, Capel B.. Temporal differences in granulosa cell specification in the ovary reflect distinct follicle fates in mice. Biol Reprod 2012;86:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers M, Morgan FH, Liew SH, Zerafa N, Gamage TU, Sarraj M, Cook M, Kapic I, Sutherland A, Scott CL. et al. PUMA regulates germ cell loss and primordial follicle endowment in mice. Reproduction 2014;148:211–219. [DOI] [PubMed] [Google Scholar]
- Naderer T, Fulcher MC.. Targeting apoptosis pathways in infections. J Leukoc Biol 2018;103:275–285. [DOI] [PubMed] [Google Scholar]
- Nakanishi T, Okamoto A, Ikeda M, Tate S, Sumita M, Kawamoto R, Tonai S, Lee JY, Shimada M, Yamashita Y.. Cortisol induces follicle regression, while FSH prevents cortisol-induced follicle regression in pigs. Mol Hum Reprod 2021;27:gaab038. [DOI] [PubMed] [Google Scholar]
- Navarro-Pando JM, Alcocer-Gómez E, Castejón-Vega B, Navarro-Villarán E, Condés-Hervás M, Mundi-Roldan M, Muntané J, Pérez-Pulido AJ, Bullon P, Wang C. et al. Inhibition of the NLRP3 inflammasome prevents ovarian aging. Sci Adv 2021;7:eabc7409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oktem O, Oktay K.. A novel ovarian xenografting model to characterize the impact of chemotherapy agents on human primordial follicle reserve. Cancer Res 2007;67:10159–10162. [DOI] [PubMed] [Google Scholar]
- Pasparakis M, Vandenabeele P.. Necroptosis and its role in inflammation. Nature 2015;517:311–320. [DOI] [PubMed] [Google Scholar]
- Pelosi E, Forabosco A, Schlessinger D.. Genetics of the ovarian reserve. Front Genet 2015;6:308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepling ME, Spradling AC.. Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev Biol 2001;234:339–351. [DOI] [PubMed] [Google Scholar]
- Peters H, Byskov AG, Himelstein-Braw R, Faber M.. Follicular growth: the basic event in the mouse and human ovary. J Reprod Fertil 1975;45:559–566. [DOI] [PubMed] [Google Scholar]
- Rea IM, Gibson DS, McGilligan V, McNerlan SE, Alexander HD, Oa R.. Age and age-related diseases: role of inflammation triggers and cytokines. Front Immunol 2018;9:586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan SLP, Knight PG, Yovich JL, Leung Y, Arfuso F, Dharmarajan A.. Granulosa cell apoptosis in the ovarian follicle—a changing view. Front Endocrinol (Lausanne) 2018;9:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi VD, Hsieh K, Munroe R, Bolcun-Filas EM, Schimenti JC.. Pharmacological inhibition of the DNA damage checkpoint prevents radiation-induced oocyte death. Genetics 2017;206:1823–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock KL, Latz E, Ontiveros F, Kono H.. The sterile inflammatory response. Annu Rev Immunol 2010;28:321–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues P, Limback D, McGinnis LK, Plancha CE, Albertini DF.. Multiple mechanisms of germ cell loss in the perinatal mouse ovary. Reproduction 2009;137:709–720. [DOI] [PubMed] [Google Scholar]
- Rucker EB, Dierisseau P, Wagner KU, Garrett L, Wynshaw-Boris A, Flaws JA, Hennighausen L.. Bcl-x and Bax regulate mouse primordial germ cell survival and apoptosis during embryogenesis. Mol Endocrinol 2000;14:1038–1052. [DOI] [PubMed] [Google Scholar]
- Safdar M, Liang A, Rajput SA, Abbas N, Zubair M, Shaukat A, Rehman AU, Jamil H, Guo Y, Ullah F.. Orexin-A regulates follicular growth, proliferation, cell cycle and apoptosis in mouse primary granulosa cells via the AKT/ERK signaling pathway. Molecules 2021;26:5635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saller S, Merz-Lange J, Raffael S, Hecht S, Pavlik R, Thaler C, Berg D, Berg U, Kunz L, Mayerhofer A.. Norepinephrine, active norepinephrine transporter, and norepinephrine-metabolism are involved in the generation of reactive oxygen species in human ovarian granulosa cells. Endocrinology 2012;153:1472–1483. [DOI] [PubMed] [Google Scholar]
- Sanchez MLE, Martinez OME, Vazquez-Nin GH.. Immunohistochemical and ultrastructural visualization of different routes of oocyte elimination in adult rats. Eur J Histochem 2012;56:102–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapuleni J, Szymanska M, Meidan R.. Diverse actions of sirtuin-1 on ovulatory genes and cell death pathways in human granulosa cells. Reprod Biol Endocrinol 2022;20:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma UC, Findlay JK, Hutt KJ.. Oocytes from stem cells. Best Pract Res Clin Obstet Gynaecol 2019;55:14–22. [DOI] [PubMed] [Google Scholar]
- Schwartz LM. Autophagic cell death during development – ancient and mysterious. Front Cell Dev Biol 2021;9:656370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Chen Y, Cheng J, Yuan S, Zhou S, Yan W, Liu J, Luo A, Wang S.. CCL5 secreted by senescent theca-interstitial cells inhibits preantral follicular development via granulosa cellular apoptosis. J Cell Physiol 2019;234:22554–22564. [DOI] [PubMed] [Google Scholar]
- Shen M, Cao Y, Jiang Y, Wei Y, Liu H.. Melatonin protects mouse granulosa cells against oxidative damage by inhibiting FOXO1-mediated autophagy: implication of an antioxidation-independent mechanism. Redox Biol 2018;18:138–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen M, Jiang Y, Guan Z, Cao Y, Li L, Liu H, Sun S-C.. Protective mechanism of FSH against oxidative damage in mouse ovarian granulosa cells by repressing autophagy. Autophagy 2017;13:1364–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng X, Liu C, Yan G, Li G, Liu J, Yang Y, Li S, Li Z, Zhou J, Zhen X. et al. The mitochondrial protease LONP1 maintains oocyte development and survival by suppressing nuclear translocation of AIFM1 in mammals. EBioMedicine 2022;75:103790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shkolnik K, Tadmor A, Ben-Dor S, Nevo N, Galiani D, Dekel N.. Reactive oxygen species are indispensable in ovulation. Proc Natl Acad Sci USA 2011;108:1462–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklan EH, Lowenthal A, Korner M, Ritov Y, Landers DM, Rankinen T, Bouchard C, Leon AS, Rice T, Rao DC. et al. Acetylcholinesterase/paraoxonase genotype and expression predict anxiety scores in health, risk factors, exercise training, and genetics study. Proc Natl Acad Sci USA 2004;101:5512–5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soleimani R, Heytens E, Darzynkiewicz Z, Oktay K.. Mechanisms of chemotherapy-induced human ovarian aging: double strand DNA breaks and microvascular compromise. Aging (Albany NY) 2011;3:782–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song ZH, Yu HY, Wang P, Mao GK, Liu WX, Li MN, Wang HN, Shang YL, Liu C, Xu ZL. et al. Germ cell-specific atg7 knockout results in primary ovarian insufficiency in female mice. Cell Death Dis 2015;6:e1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer JM, Western PS.. A step toward making human oocytes. Nat Biotechnol 2019;37:24–25. [DOI] [PubMed] [Google Scholar]
- Stringer JM, Winship A, Liew SH, Hutt K.. The capacity of oocytes for DNA repair. Cell Mol Life Sci 2018;75:2777–2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer JM, Winship A, Zerafa N, Wakefield M, Hutt K.. Oocytes can efficiently repair DNA double-strand breaks to restore genetic integrity and protect offspring health. Proc Natl Acad Sci USA 2020;117:11513–11522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strowig T, Henao-Mejia J, Elinav E, Flavell R.. Inflammasomes in health and disease. Nature 2012;481:278–286. [DOI] [PubMed] [Google Scholar]
- Suen DF, Norris KL, Youle RJ.. Mitochondrial dynamics and apoptosis. Genes Dev 2008;22:1577–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh E-K, Yang A, Kettenbach A, Bamberger C, Michaelis AH, Zhu Z, Elvin JA, Bronson RT, Crum CP, McKeon F.. p63 protects the female germ line during meiotic arrest. Nature 2006;444:624–628. [DOI] [PubMed] [Google Scholar]
- Sun YC, Wang YY, Sun XF, Cheng SF, Li L, Zhao Y, Shen W, Chen H.. The role of autophagy during murine primordial follicle assembly. Aging 2018;10:197–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Ma L, Guo S, Liang M, Jiang Z.. High doses of FSH induce autophagy in bovine ovarian granulosa cells via the AKT/mTOR pathway. Reprod Domest Anim 2021a;56:324–332. [DOI] [PubMed] [Google Scholar]
- Tang Z, Zhang Z, Lin Q, Xu R, Chen J, Wang Y, Zhang Y, Tang Y, Shi C, Liu Y. et al. HIF-1α/BNIP3-mediated autophagy contributes to the luteinization of granulosa cells during the formation of corpus luteum. Front Cell Dev Biol 2021b;619924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatone C, Amicarelli F, Carbone MC, Monteleone P, Caserta D, Marci R, Artini PG, Piomboni P, Focarelli R.. Cellular and molecular aspects of ovarian follicle ageing. Hum Reprod Update 2008;14:131–142. [DOI] [PubMed] [Google Scholar]
- Thorburn A. Apoptosis and autophagy: regulatory connections between two supposedly different processes. Apoptosis 2008;13:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y-Q, Li X-L, Wang W-J, Hao H-S, Zou H-Y, Pang Y-W, Zhao X-M, Zhu H-B, Du W-H.. Knockdown of bone morphogenetic protein 4 gene induces apoptosis and inhibits proliferation of bovine cumulus cells. Theriogenology 2022;188:28–36. [DOI] [PubMed] [Google Scholar]
- Titus S, Li F, Stobezki R, Akula K, Unsal E, Jeong K, Dickler M, Robson M, Moy F, Goswami S.. Impairment of BRCA1-related DNA double-strand break repair leads to ovarian aging in mice and humans. Sci Transl Med 2013;5:172ra121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titus S, Szymanska KJ, Musul B, Turan V, Taylan E, Garcia-Milian R, Mehta S, Oktay K.. Individual-oocyte transcriptomic analysis shows that genotoxic chemotherapy depletes human primordial follicle reserve in vivo by triggering proapoptotic pathways without growth activation. Sci Rep 2021;11:407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traut MH, Berg D, Berg U, Mayerhofer A, Kunz L.. Identification and characterization of Ca2+-activated K+ channels in granulosa cells of the human ovary. Reprod Biol Endocrinol 2009;7:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui K-H, Wang P-H, Lin L-T, Li C-J.. DHEA protects mitochondria against dual modes of apoptosis and necroptosis in human granulosa HO23 cells. Reproduction 2017;154:101–110. [DOI] [PubMed] [Google Scholar]
- Uda M, Ottolenghi C, Crisponi L, Garcia JE, Deiana M, Kimber W, Forabosco A, Cao A, Schlessinger D, Pilia G.. Foxl2 disruption causes mouse ovarian failure by pervasive blockage of follicle development. Hum Mol Genet 2004;13:1171–1181. [DOI] [PubMed] [Google Scholar]
- Ullah S, Zhang M, Yu H, Mustafa S, Shafiq M, Wei Q, Wang W, Jan M, Mao D.. Heat exposure affected the reproductive performance of pregnant mice: enhancement of autophagy and alteration of subcellular structure in the corpus luteum. Reprod Biol 2019;19:261–269. [DOI] [PubMed] [Google Scholar]
- Umehara T, Winstanley YE, Andreas E, Morimoto A, Williams EJ, Smith KM, Carroll J, Febbraio MA, Shimada M, Russell DL. et al. Female reproductive life span is extended by targeted removal of fibrotic collagen from the mouse ovary. Sci Adv 2022;8:eabn4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urra J, Blohberger J, Tiszavari M, Mayerhofer A, Lara HE.. In vivo blockade of acetylcholinesterase increases intraovarian acetylcholine and enhances follicular development and fertility in the rat. Sci Rep 2016;6:30129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaithiyanathan K, Liew SH, Zerafa N, Gamage T, Cook M, O’Reilly LA, Bouillet P, Scott CL, Strasser A, Findlay JK. et al. Bcl2-modifying factor promotes germ cell loss during murine oogenesis. Reproduction 2016;151:553–562. [DOI] [PubMed] [Google Scholar]
- Valckx SD, Arias-Alvarez M, D, Pauw I, Fievez V, Vlaeminck B, Fransen E, Bols PE, L Jl. Fatty acid composition of the follicular fluid of normal weight, overweight and obese women undergoing assisted reproductive treatment: a descriptive cross-sectional study. Reprod Biol Endocrinol 2014;12:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaskivuo TE, Tapanainen JS.. Apoptosis in the human ovary. Reprod Biomed Online 2003;6:24–35. [DOI] [PubMed] [Google Scholar]
- Wajant H, Siegmund D.. TNFR1 and TNFR2 in the control of the life and death balance of macrophages. Front Cell Dev Biol 2019;7:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace WH, Kelsey TW.. Human ovarian reserve from conception to the menopause. PLoS One 2010;5:e8772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Youle RJ.. The role of mitochondria in apoptosis. Annu Rev Genet 2009;43:95–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Xue X, Wang Y, Zhao H, Zhang Y, Wang H, Miao D.. BMI1 deficiency results in female infertility by activating p16/p19 signaling and increasing oxidative stress. Int J Biol Sci 2019;15:870–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Yang H, Fu Y, Teng X, Wang C, Xu W.. The key role of peroxisomes in follicular growth, oocyte maturation, ovulation, and steroid biosynthesis. Oxid Med Cell Longev 2022;2022:7982344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Kim NS, Haince J-F, Kang HC, David KK, Andrabi SA, Poirier GG, Dawson VL, Dawson TM.. Poly (ADP-ribose)(PAR) binding to apoptosis-inducing factor is critical for par polymerase-1–dependent cell death (parthanatos). Sci Signal 2011;4:ra20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Li C, Ali I, Li L, Wang G.. N-acetylcysteine modulates non-esterified fatty acid-induced pyroptosis and inflammation in granulosa cells. Mol Immunol 2020;127:157–163. [DOI] [PubMed] [Google Scholar]
- Wang Y-Y, Sun Y-C, Sun X-F, Cheng S-F, Li B, Zhang X-F, De Felici M, Shen W.. Starvation at birth impairs germ cell cyst breakdown and increases autophagy and apoptosis in mouse oocytes. Cell Death Dis 2017;8:e2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe R, Kimura N.. Non-suckling starvation of neonatal mice promotes primordial follicle formation with activation of ovarian autophagy. J Reprod Dev 2018;64:89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winship AL, Alesi LR, Sant S, Stringer JM, Cantavenera A, Hegarty T, Requesens CL, Liew SH, Sarma U, Griffiths MJ. et al. Checkpoint inhibitor immunotherapy diminishes oocyte number and quality in mice. Nat Cancer 2022;3:1–13. [DOI] [PubMed] [Google Scholar]
- Winship AL, Stringer JM, Liew SH, Hutt KJ.. The importance of DNA repair for maintaining oocyte quality in response to anti-cancer treatments, environmental toxins and maternal ageing. Hum Reprod Update 2018;24:119–134. [DOI] [PubMed] [Google Scholar]
- Won J-H, Park S, Hong S, Son S, Yu J-W.. Rotenone-induced impairment of mitochondrial electron transport chain confers a selective priming signal for NLRP3 inflammasome activation. J Biol Chem 2015;290:27425–27437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X, Wang X, Zheng Y, Jiang J, Hu J.. What role does pyroptosis play in microbial infection? J Cell Physiol 2019;234:7885–7892. [DOI] [PubMed] [Google Scholar]
- Xie F, Zhang J, Zhai M, Liu Y, Hu H, Yu Z, Zhang J, Lin S, Liang D, Cao Y.. Melatonin ameliorates ovarian dysfunction by regulating autophagy in PCOS via the PI3K-AKT pathway. Reproduction 2021;162:73–82. [DOI] [PubMed] [Google Scholar]
- Yang X, Wu LL, Chura LR, Liang X, Lane M, Norman RJ, Robker RL.. Exposure to lipid-rich follicular fluid is associated with endoplasmic reticulum stress and impaired oocyte maturation in cumulus–oocyte complexes. Fertil Steril 2012;97:1438–1443. [DOI] [PubMed] [Google Scholar]
- Yao J, Huang R, Li M, Jiang Y, Wu P, Li Y, Peng W, Hua C, Huang Y, You H. et al. PTEN expression in human granulosa cells is associated with ovarian responses and clinical outcomes in IVF. Reprod Sci 2021;28:1910–1921. [DOI] [PubMed] [Google Scholar]
- Yefimova MG, Lefevre C, Bashamboo A, Eozenou C, Burel A, Lavault MT, Meunier AC, Pimentel C, Veau S, Neyroud AS. et al. Granulosa cells provide elimination of apoptotic oocytes through unconventional autophagy-assisted phagocytosis. Hum Reprod 2020;35:1346–1362. [DOI] [PubMed] [Google Scholar]
- You SY, Park YS, Jeon HJ, Cho DH, Jeon HB, Kim SH, Chang JW, Kim JS, Oh JS.. Beclin-1 knockdown shows abscission failure but not autophagy defect during oocyte meiotic maturation. Cell Cycle 2016;15:1611–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu P, Zhang X, Liu N, Tang L, Peng C, Chen X.. Pyroptosis: mechanisms and diseases. Signal Transduct Target Ther 2021;6:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S-W, Wang H, Dawson TM, Dawson VL.. Poly (ADP-ribose) polymerase-1 and apoptosis inducing factor in neurotoxicity. Neurobiol Dis 2003;14:303–317. [DOI] [PubMed] [Google Scholar]
- Yuan HJ, Li ZB, Zhao XY, Sun GY, Wang GL, Zhao YQ, Zhang M, Tan JH.. Glucocorticoids impair oocyte competence and trigger apoptosis of ovarian cells via activating the TNF-α system. Reproduction 2020;160:129–140. [DOI] [PubMed] [Google Scholar]
- Zhang H, Baehrecke EH.. Eaten alive: novel insights into autophagy from multicellular model systems. Trends Cell Biol 2015;25:376–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Liu L, Li X, Busayavalasa K, Shen Y, Hovatta O, Gustafsson JA, Liu K.. Life-long in vivo cell-lineage tracing shows that no oogenesis originates from putative germline stem cells in adult mice. Proc Natl Acad Sci USA 2014;111:17983–17988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng WJ, Zhang H, Gorre N, Risal S, Shen Y, Liu K.. Two classes of ovarian primordial follicles exhibit distinct developmental dynamics and physiological functions. Hum Mol Genet 2014;23:920–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhihan T, Xinyi M, Qingying L, Rufei G, Yan Z, Xuemei C, Yanqing G, Yingxiong W, Junlin H.. Autophagy participates in cyst breakdown and primordial folliculogenesis by reducing reactive oxygen species levels in perinatal mouse ovaries. J Cell Physiol 2019;234:6125–6135. [DOI] [PubMed] [Google Scholar]
- Zhou J, Yao W, Li C, Wu W, Li Q, Liu H.. Administration of follicle-stimulating hormone induces autophagy via upregulation of HIF-1α in mouse granulosa cells. Cell Death Dis 2017;8:e3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann M. Neuronal ache splice variants and their non-hydrolytic functions: redefining a target of ache inhibitors? Br J Pharmacol 2013;170:953–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analysed in support of this research.