Abstract
Background
Glioblastoma (GBM) is a fast-growing primary brain tumor characterized by high invasiveness and resistance. This results in poor patient survival. Resistance is caused by many factors, including cell-extracellular matrix (ECM) interactions. Here, we addressed the role of adhesion protein integrin α2, which we identified in a high-throughput screen for novel potential targets in GBM cells treated with standard therapy consisting of temozolomide (TMZ) and radiation.
Methods
In our study, we used a range of primary/stem-like and established GBM cell models in vitro and in vivo. To identify regulatory mechanisms, we employed high-throughput kinome profiling, Western blotting, immunofluorescence staining, reporter, and activity assays.
Results
Our data showed that integrin α2 is overexpressed in GBM compared to normal brain and, that its deletion causes radiochemosensitization. Similarly, invasion and adhesion were significantly reduced in TMZ-irradiated GBM cell models. Furthermore, we found that integrin α2-knockdown impairs the proliferation of GBM cells without affecting DNA damage repair. At the mechanistic level, we found that integrin α2 affects the activity of activating transcription factor 1 (ATF1) and modulates the expression of extracellular signal-regulated kinase 1 (ERK1) regulated by extracellular signals. Finally, we demonstrated that integrin α2-deficiency inhibits tumor growth and thereby prolongs the survival of mice with orthotopically growing GBM xenografts.
Conclusions
Taken together our data suggest that integrin α2 may be a promising target to overcome GBM resistance to radio- and chemotherapy. Thus, it would be worth evaluating how efficient and safe the adjuvant use of integrin α2 inhibitors is to standard radio(chemo)therapy in GBM.
Keywords: ATF1, ERK1, glioblastoma, integrin α2, radiochemoresistance
Graphical Abstract
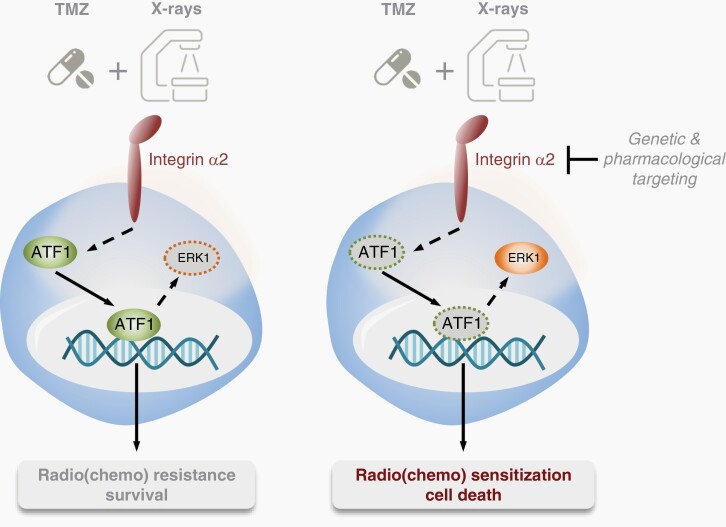
Graphical Abstract.
Key Points.
Integrin α2-targeting sensitizes GBM cells to radio- and chemotherapy.
Integrin α2-targeting results in decreased ATF1 activity in GBM cells.
Importance of the Study.
The interaction of GBM cells with the extracellular matrix (ECM) plays a central role in tumor resistance to radio- and chemotherapy. The adhesion receptor integrin α2 is more highly expressed in GBM compared to normal tissue; however, its role in GBM progression and therapy resistance remains largely unknown. In this study, we demonstrated that the knockdown of integrin α2 decreases protein levels and activity of the transcription factor ATF1, resulting in decreased proliferation and reduced survival of GBM cells in response to radio(chemo)therapy in vitro and in vivo. Our data indicate that integrin α2 is a potential target that could be adjuvantly inhibited to standard radio(chemo)therapy in GBM.
Glioblastoma (GBM) belongs to the group of high-grade gliomas and represents the most common primary brain tumor in adults.1 To date, the combination of maximal surgical resection, radiotherapy, and chemotherapy with the DNA alkylating agent temozolomide (TMZ) is the standard of care for patients with GBM. Yet, the prognosis of patients with GBM remains poor with a median survival time between 11 and 16 months.2,3 Characteristic features of GBM cells are remarkable resistance to radio(chemo)therapy as well as diffuse and destructive infiltration of the normal brain.4,5 Despite extensive studies aimed at better understanding the molecular determinants of these traits, including genetic, epigenetic, and microenvironmental factors, the full molecular mechanisms remain largely to be unraveled. However, only a mechanistic understanding provides the basis for the development of novel, molecular-targeted therapeutic interventions.6
In addition to a myriad of druggable cytoplasmic kinases, cell membrane receptors like receptor tyrosine kinases or integrins represent promising cancer targets. Integrins are heterodimers consisting of α and β subunits. Till date, 18 α and 8 β chains generating 24 different integrin adhesion receptors are known.7 Both, the functional properties of cancer cells and therapy resistance are essentially determined by the adhesion of cancer cells to the extracellular matrix (ECM) through receptors of the integrin family.7,8 Similar to other integrin subunits, integrin α2 is overexpressed in various cancer types, e.g., head and neck cancer, pancreatic cancer, acute myeloid leukemia, gastric cancer, and colorectal cancer.9–13 Its contribution to tumor growth and metastasis has been shown to involve multiple signaling pathways such as STAT3, YAP, Akt/FoxO1, and RhoA-p38 MAPK.14–17 Consistently, elevated levels of integrin α2 contribute to the development of chemoresistance.16,18 Despite a recent report by Guo and coworkers about the potential function of integrin α2 in GBM cell migration,19 the mechanistic role of integrin α2 in therapy resistance, particularly for standard clinically applied radio(chemo)therapy regimes, is largely unknown.
Here, we investigated the role of integrin α2 in GBM cell models in vitro and in vivo exposure to the standard of care TMZ/radiotherapy combination. We demonstrated that integrin α2-targeting mediates antimitogenic effects in GBM cells. In GBM cultures and in orthotopic GBM xenografts, we revealed integrin α2 inhibition to elicit sensitization to radio- and chemotherapy. Mechanistically, this was associated with reduced activity of activating transcription factor 1 (ATF1). Overall, our data suggest that integrin α2 is an attractive therapeutic target that may be adjuvantly inhibited together with standard radio(chemo)therapy, and propose a new molecular mechanism by which integrin α2 contributes to the regulation of GBM radio(chemo)resistance.
Materials and Methods
Cell Culture
Human GBM cell lines A172 (Cat# CRL-1620), LN229 (Cat# CRL-2611), U87MG (HTB-14), and U138MG (Cat# HTB-16) were purchased from American Type Culture Collection (ATCC). U343MG, DDHT7607, and DD-T4 cell lines were kindly provided by A. Temme (University Hospital Dresden, Germany), U251MG and LN405 cell lines by L. Kunz-Schughart (University Hospital Dresden, Germany), and patient-derived human GBM stem-like cells GS-8 by K. Lamszus (University Medical Center Hamburg-Eppendorf, Germany) via material transfer agreement. GS-8_GFP/fLuc cells were generated by lentiviral transduction with the vector p6NST50 containing the firefly luciferase gene as published.20 A172, LN229, LN405, DD-T4, DDHT7606, U251MG, U138MG, and U87MG cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) high glucose (Sigma–Aldrich) with 10% heat-inactivated fetal bovine serum (Sigma–Aldrich), 2 mM l-glutamine (Thermo Fischer Scientific) and 1 × MEM non-essential amino acids (Sigma–Aldrich) at 37°C in a humidified 8.5% CO2 incubator. U343MG cell line was maintained in Basal Medium Eagle (BME) (Thermo Fischer Scientific) supplemented with 10% heat-inactivated fetal bovine serum (Sigma–Aldrich), 1x MEM non-essential amino acids (Sigma–Aldrich), 2 mM l-glutamine (Thermo Fischer Scientific) and 10 mM HEPES (Sigma–Aldrich) at 37°C in a humidified 5% CO2 incubator. Stem-like cells GS-8 and GS-8_GFP/fLuc were cultured in neurobasal medium (NBM) (Thermo Fischer Scientific) with 2 mM l-glutamine (Thermo Fischer Scientific), 1× B27 supplement (Thermo Fischer Scientific), 32 U/mL Heparin (Millipore), 20 ng/mL FGF (Novus) and 20 ng/mL EGF (Novus) at 37°C in a humidified 5% CO2 incubator. All cells are authenticated by STR profiling and were regularly tested to ensure the absence of mycoplasma.
shRNA-Mediated Gene Knockdown
Details regarding plasmids and experimental procedures are available in Supplementary Methods.
High-Throughput siRNA Screen
A total of 50 000 human GBM cells per well were plated overnight on 12-well plates and transfected with the ON-TARGETplus SMARTpool siRNA library (Horizon Discovery) by the use of Lipofectamin RNAiMAX (Thermo Fischer Scientific) according to the manufacturer`s protocol. After 48 h, transfected cells were plated for colony formation as further described in Supplementary Methods. The results of the high-throughput siRNA screen are displayed as enhancement ratios (an average plating efficiency upon control siRNA transfection divided by an average plating efficiency upon target siRNA transfection).
Analysis Using Online Databases
Detailed information regarding data analysis is provided in Supplementary Methods.
In Vitro Assays
Detailed information regarding in vitro cell proliferation, adhesion assay, 3D invasion assay, and foci assay are included in Supplementary Methods.
Animal Studies
All animal experiments were approved by Landesdirektion Sachsen, Germany (TVV 27/2019) and performed in accordance with the German and Saxony animal welfare guidelines. For further information, see Supplementary Methods.
Histology of Orthotopic GBM Xenografts
Details regarding immunofluorescence analysis of mouse brain sections are shown in Supplementary Methods.
Protein Kinase Activity Profiling
Kinase activities were measured using PamGene Technology (Genomics and Proteomics Core Facility, DKFZ, Heidelberg, Germany) as published.21 Further information is included in Supplementary Methods.
Western Blotting
Detailed information regarding Western blotting is provided in Supplementary Methods.
ATF1 Transcription Factor Activity Assay
Analysis of ATF1 activity was performed using a commercially available ELISA kit (Abbexa) according to the manufacturer’s manual.
Transient Plasmid Transfections
Details regarding plasmids and experimental procedures are available in Supplementary Methods.
Quantitative Real-Time PCR
Details regarding experimental procedures and primer sequences are provided in Supplementary Methods.
Dual-Luciferase Reporter Assay
Detailed information regarding the luciferase reporter assay is provided in Supplementary Methods.
Statistical Analysis
Data are expressed as mean values ± SEM of at least 3 independent experiments. For statistical analysis of 2 groups, a two-tailed unpaired Student’s t-test was applied. Comparisons of >2 groups were performed with ANOVA (followed by Sidak or Dunnett post hoc test). Statistical significance of animal experiments was tested using Gehan-Breslow-Wilcoxon test. Statistical analysis was performed in GraphPad Prism 7 (GraphPad Prism Software Inc). P values are depicted as: *P < .05; **P < .01; ***P < .001, ****P < .0001.
Results
High-Throughput siRNA Screen Identifies Integrin α2 as a Critical Determinant of GBM Cell Radio(chemo)resistance
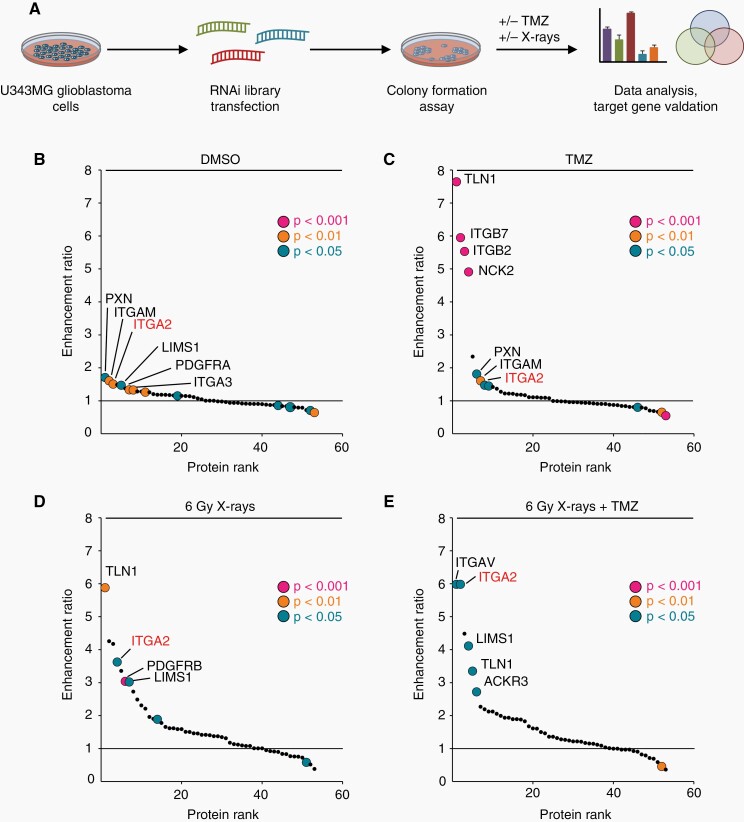
In our search for novel potential cancer targets in focal adhesions, we commenced our study by designing a siRNA library targeting 53 genes encoding various focal adhesion proteins as well as receptors for growth factors and cytokines (Figure 1A). We tested for radio(chemo)sensitization using a clonogenic survival assay with U343MG cell line as a representative GBM cell model. We observed integrin α2-targeting significantly impairing basal U343MG cell survival and enhancing sensitivity towards TMZ and irradiation (IR) (Figure 1B–D). Intriguingly, cells depleted of integrin α2, integrin αV, C-X-C chemokine receptor type 7 (CXCR7), Talin1, or LIM and senescent cell antigen-like-containing domain protein 1 (LIMS1) revealed the strongest reduction in survival (expressed as enhancement ratios) after 6 Gy X-ray/TMZ co-treatment relative to controls indicating integrin α2 as a highly promising candidate for GBM treatment (Figure 1E).
Figure 1.
High-throughput siRNA screening identifies new regulators of radiochemoresistance in human GBM cells. (A) U343MG cells were transfected with siRNAs targeting 53 focal adhesion proteins, receptor tyrosine kinases and chemokine receptors, and seeded for colony formation assay. Cells were treated with either 0.3 µM TMZ (DMSO as a control), 6 Gy X-rays (0 Gy as a control) alone, or in combination. (B), (C), (D), (E) Clonogenic survival of transfected U343MG cells upon indicated treatments was assessed. Data are presented as enhancement ratios (an average plating efficiency upon control siRNA treatment divided by an average plating efficiency upon target siRNA treatment). Sensitizing enhancement ratios of cells treated with control siRNA were set as 1 (black line), n = 3. Statistics of raw data were performed with unpaired two-tailed Student’s t-test.
Integrin α2-Depletion Blocks GBM cell Proliferation and Spreading In Vitro but is Dispensable for DNA Damage Repair
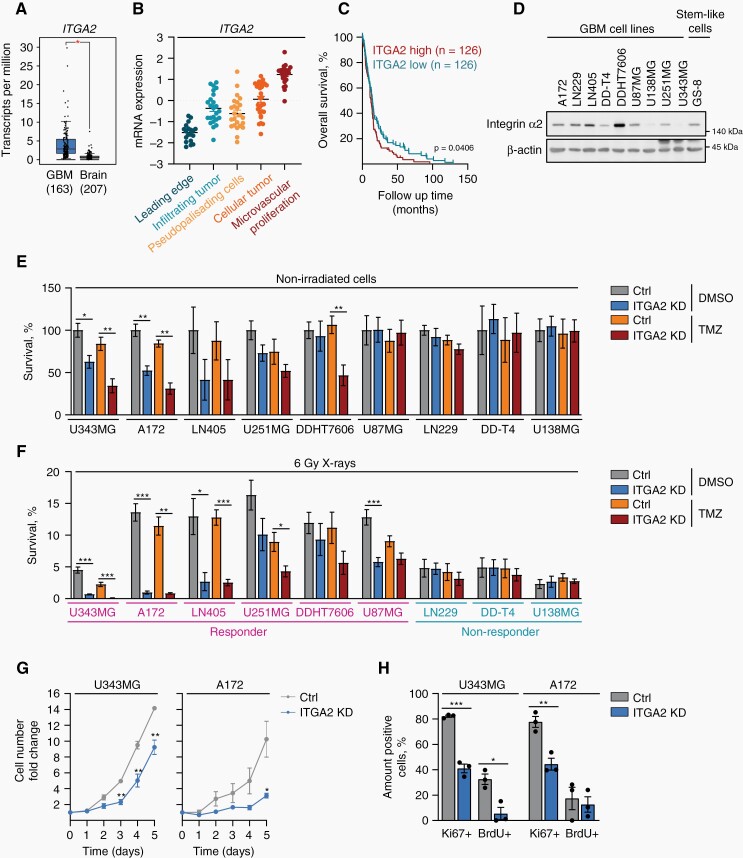
To correlate our in vitro findings with clinical data, we comparatively analyzed ITGA2 mRNA expression in tumor samples and normal brains using the GEPIA web-based tool.22ITGA2 mRNA was significantly overexpressed in GBM relative to healthy brain and associated with tumor recurrence (Figure 2A, Supplementary Figure 1A, B). According to the Ivy GAP dataset, ITGA2 levels were highest in the microvascular proliferation area (Figure 2B). Furthermore, high, in contrast to low, ITGA2 expression caused significantly less overall GBM patient survival (Figure 2C) (R2: Genomics Analysis and Visualization Platform).
Figure 2.
Integrin α2-targeting effectively radiochemosensitizes GBM cells. (A) Comparative mRNA expression analysis of ITGA2 between GBM and normal brain using the GEPIA database (http://gepia.cancer-pku.cn). One-way ANOVA (P value cutoff .01). (B) ITGA2 mRNA expression in indicated anatomical structures from the Ivy GAP database. Each point corresponds to an individual laser-microdissected sample. (C) Graphs illustrating the correlation between overall survival and ITGA2 mRNA expression levels in GBM patients using Tumor Glioblastoma-TCGA-540-MAS5.0-u133a database (Log-rank test). (D) Western blot analysis for integrin α2 expression (β-actin as a loading control) from GBM cell lines and stem-like GS-8 cells. (E), (F) Analysis of the radiochemosensitizing potential of ITGA2 siRNA-mediated knockdown in 9 human GBM cell lines exposed to TMZ (DMSO as a control) and 6 Gy X-ray irradiation determined as clonogenic survival. Survival of control nonirradiated DMSO-treated cells was set as 100%, n = 3–6 (responders to TMZ and/or X-rays are highlighted in pink). (G) Growth curves of integrin α2-deficient and control A172 and U343MG cell cultures over 5 days, n = 3. (H) Percentages of BrdU+ and Ki67+ cells in integrin α2-deficient and control U343MG and A172 cells on day 1 after plating, n = 3. All data are presented as mean ± SEM with individual values. Statistical significance was analyzed with unpaired two-tailed Student’s t-test, *P < .05, **P < .01, ***P < .001.
To test for the generality of integrin α2-depletion-mediated radio(chemo)sensitization, we next employed a panel of established and stem-like, integrin α2-expressing GBM cell models (Figure 2D). While 4 out of 9 GBM models showed reduced basal survival upon integrin α2-knockdown, 6 (defined as “responder”) out of 9 were sensitized to either TMZ and/or X-rays (Figure 2E, F, Supplementary Figure 1C). These variable survival responses might be due to differences in genetic/epigenetic backgrounds and/or multiple escape mechanisms activated in response to treatment.
Subsequently, we evaluated additional key tumor-associated functions of integrin α2 including proliferation, invasion, adhesion, and DNA damage repair. We first found reduced proliferation capacities of integrin α2-deficient U343MG and A172 cells (Figure 2G and H, Supplementary Figure 1D). Likewise, the adhesion of U138MG and A172 cells to type I collagen, a ligand of the collagen I receptor integrin α2β1, was significantly compromised by silencing integrin α2 (Supplementary Figure 1E). Invasion capabilities in 3D type I collagen were similarly diminished in radiosensitized cell models (responders: U343MG, A172) in contrast to a nonradiosensitized model (nonresponder: U138MG) (Supplementary Figure 1F, G). Of note, the treatment of these tested GBM cell models with TMZ and/or X-rays failed to alter their invasive capability indicating that current conventional radio(chemo)therapy seems to be incapable of deactivating adhesion- and invasion-related processes. Finally, we investigated the repair of DNA double-strand breaks (DSB) presenting as the most life-threatening DNA damage events.23 Based on the observed radiochemosensitization, we surprisingly observed similar DSB repair kinetics, measured by immunofluorescence staining for γH2A.X and 53BP1 between integrin α2-deficient and control cells (Supplementary Figure 2).
Altogether, we conclude that the loss of integrin α2 in GBM cells impairs cell proliferation, adhesion, and invasion while leaving DSB repair unchanged.
Integrin α2-Deficiency Delays Growth of Orthotopic GBM Xenografts and Prolongs Survival in Combination with Radiochemotherapy
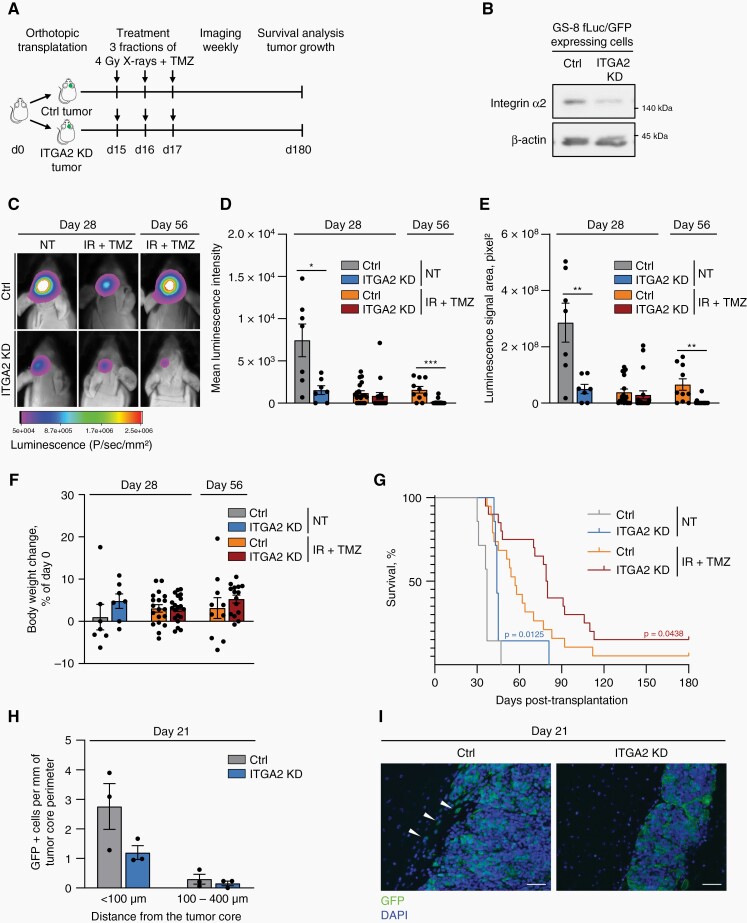
To further prove whether integrin α2 plays an essential role in radiochemoresistance, we employed our previously documented orthotopic GBM xenograft model characterized by invasiveness, excessive vascularization, and pronounced necrotic core.24 Two weeks after implantation of integrin α2-deficient or control, stem-like GS-8 GBM cells expressing EGFP and firefly luciferase into the right mouse brain hemisphere, tumor-bearing animals received 3 cycles of radiochemotherapy (4 Gy X-rays/TMZ) (Figure 3A, B). Luminescence imaging revealed significant growth delay in integrin α2-deficient tumors from day 28 and day 56 after implantation relative to controls (Figure 3C–E). We observed neither critical nor significant alterations in body weight due to integrin α2-knockdown or treatment (Figure 3F). Intriguingly, mice with integrin α2-deficient tumors revealed significant prolongation in their overall survival when exposed to radiochemotherapy (Figure 3G). To gain insight into invasive behavior, we assessed the amount of GBM cells in the brain parenchyma, which showed reduction upon integrin α2-knockdown (Figure 3H, I). Taken together, our data provide evidence that integrin α2 plays a key role in tumor growth and response to current standard radiotherapy/TMZ treatment in vivo.
Figure 3.
Integrin α2-knockdown delays tumor growth and increases survival in vivo. (A) Graph illustrating the treatment scheme. GS-8 fLuc/GFP integrin α2-deficient or control cells were orthotopically transplanted into mice. Mice were treated with TMZ (22mg/kg)/4 Gy X-rays or 0.9% NaCl solution (as a control) on 3 consecutive days. (B) Representative Western blot showing integrin α2-knockdown efficiency in integrin α2-deficient and control GS-8 fLuc/GFP cells. (C) In vivo luminescence imaging of tumor-bearing mice on day 28 and day 56. (D), (E) Assessment of luminescence signal in indicated treatment groups on days 28 and 56 after transplantation, n = 7–20 mice per group. (F) Analysis of body weight changes in mice bearing integrin α2-deficient or control tumors on days 28 and 56 after transplantation upon indicated treatments, n = 7–20 mice per group. (G) Kaplan–Meier curves demonstrate the survival of mice with integrin α2-deficient or control tumors treated as indicated, n = 7 mice in untreated groups (NT), n = 19–20 mice in IR + TMZ treated groups. (H), (I) Graph and corresponding images show the number of GS-8 fLuc/GFP integrin α2-deficient or control cells invading the normal brain on day 21 after transplantation. Scale bar (H), 50 µm. All data are shown as mean ± SEM. Statistics were performed using an unpaired two-tailed Student’s t-test. For analysis of survival curves Gehan–Breslow–Wilcoxon test was applied, *P < .05, **P < .01, ***P < .001.
The Serine/Threonine Kinome, Particularly the MAPK Signaling Pathway, is Affected in Integrin α2-Depleted GBM Cells
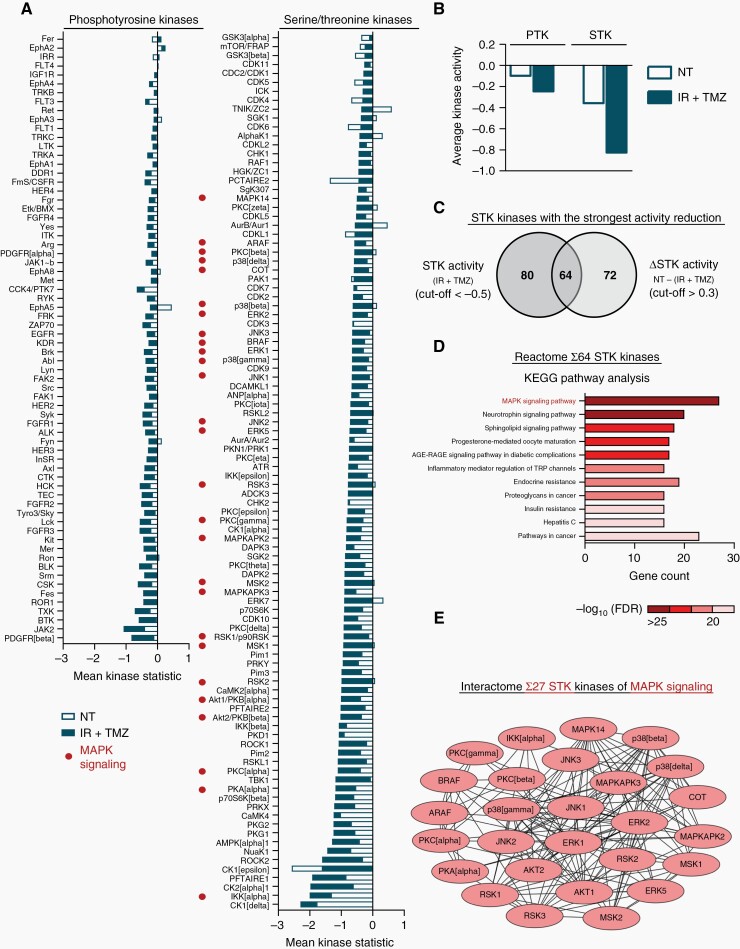
To better understand the underlying mechanisms of how integrin α2-depletion elicits radiochemosensitization in GBM cells, we determined activity profiles of phosphotyrosine (PTK) and serine/threonine (STK) kinases using Pamgene technology. Our data revealed that phosphorylation of PTK bait peptides shows only marginal changes upon integrin α2-depletion resulting in subtle alterations in PTK activity profile (maximum reduction of 0.8-fold) relative to controls (Figure 4A, Supplementary Figure 3A, B). At the same time, STK-associated bait peptide phosphorylation and STK activities demonstrated a 2-fold decrease in integrin α2-deficient U343MG cells upon 6 Gy X-rays/TMZ as compared with controls (Figure 4A, Supplementary Figure 3A, B). These data suggest that the radiochemosensitizing effect of integrin α2-deficiency is primarily associated with a reduction in serine/threonine rather than phosphotyrosine kinome (Figure 4B). Remarkably, the majority of STK deregulated upon integrin α2-knockdown belong to the AGC kinase subfamily (Supplementary Figure 3C) involved in multiple cellular processes such as proliferation, metabolism, and protein synthesis.25 To determine which pathway might be preferentially affected in integrin-α2-deficient GBM cells under 6 Gy X-ray/TMZ administration, we performed Reactome (KEGG database) and interactome analysis (STRING database) of the 64 STK kinases that exhibited the most severe changes upon treatment compared with untreated controls (Figure 4C). Our data revealed significant enrichment in the mitogen-activated protein kinase (MAPK) signaling pathway (Figure 4D, E).
Figure 4.
Integrin α2-knockdown modulates kinome profiles of GBM cells under radiochemotherapy. (A), (B) Diagrams illustrate predicted changes in activities of individual phosphotyrosine kinases (PTK) and serine/threonine kinases (STK) as well as overall alterations in STK- and PTK-profiles in integrin α2-deficient U343MG cells relative to controls upon irradiation (IR) + temozolomide (TMZ) or non-treated (NT) controls. Mean Kinase Statistic values of 3 independent experiments demonstrate changes in kinase activities. (C) Venn diagram of top 64 STK with the strongest activity reduction in integrin α2-deficient cells under IR + TMZ (Mean Kinase Statistic cutoff ≤ −0.5) in comparison to the untreated group (Δ Mean Kinase Statistic NT – (IR + TMZ) cutoff > 0.3). (D) KEGG pathway enrichment analysis including the top 64 deregulated STK identified in C. (E) Interaction analysis of 27 STK kinases associated with MAPK signaling pathway and deregulated in integrin α2-deficient cells upon IR + TMZ.
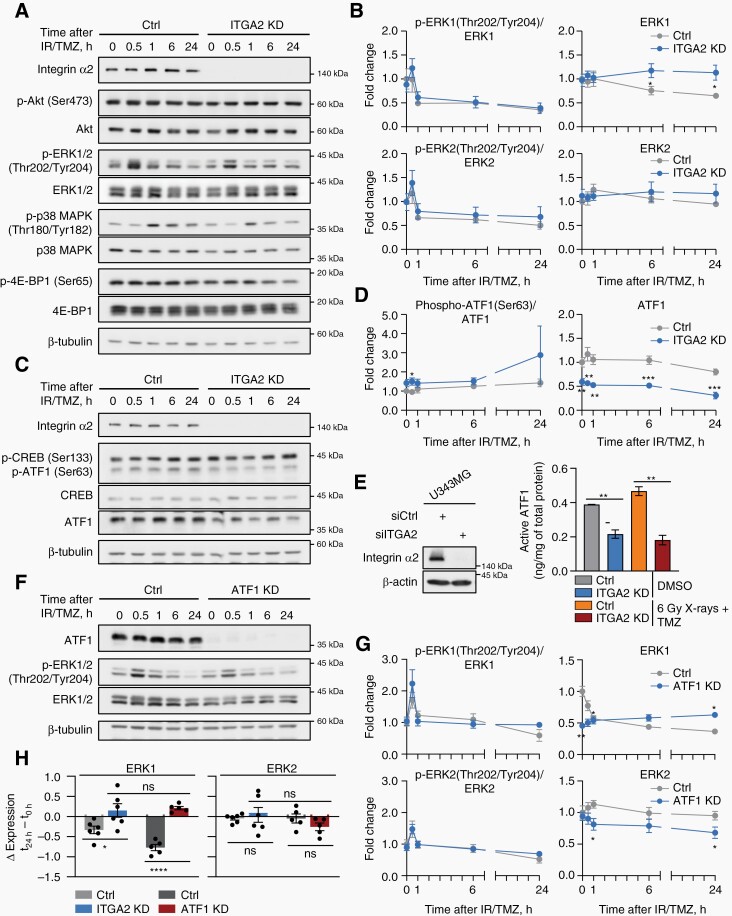
Given these findings, we next assessed the phosphorylation of key components of the MAPK signaling pathway (e.g., ERK1/2, p38) as well as Akt, reported to be associated with integrin α2.16 Six hours after 6 Gy X-rays/TMZ, we observed only moderate reduction in Ser473-Akt phosphorylation upon integrin α2-silencing and no significant changes in total Akt, phosphorylated forms of ERK1/2 (Thr202/Tyr204) and p38 (Thr180/Tyr182) (Figure 5A, B, Supplementary Figure 4A). Additionally, we exhibited significant induction of total ERK1 but not ERK2 relative to controls. To take a closer look at the ERK1/2 and Akt signaling pathways, we analyzed selected common downstream targets. Translation initiation repressor 4E-BP1 is integral to both signaling pathways and is associated with the maintenance of the transformed phenotype and resistance to therapy.26 CREB and ATF1 are members of the CREB family of transcription factors and are overexpressed in GBM (https://www.proteinatlas.org).27,28 We found significantly reduced 4E-BP1 (Ser65) phosphorylation in integrin α2-deficient U343MG cells 24 h after 6 Gy X-rays/TMZ (Figure 5A, Supplementary Figure 4A). Intriguingly and in contrast to CREB, total ATF1 levels and its activity significantly decreased upon integrin α2-silencing, which remained stable throughout the 24-h observation period. The generality of this result was demonstrated in our panel of GBM cell lines (Figure 5C–E, Supplementary Figure 5A). Of note, the phosphorylation levels of both ATF1 and CREB showed only marginal and nonsignificant changes, respectively (Figure 5C, D). Finally, we addressed the question of the hierarchical positioning amongst ATF1, ERK1, and ERK2. We observed similarity in ERK1 expression upon integrin α2- and ATF1-depletion, while ERK2 levels remained unaltered (Figure 5F–H). Together, these data suggest that the transcription factor ATF1 and the signaling mediator ERK1 play a critical role in the integrin α2-mediated response of GBM cells to radiochemotherapy.
Figure 5.
Integrin α2-deficiency regulates protein levels of ERK1 and ATF1 in GBM cells. Representative Western blots (A), (C) and densitometry analysis (B), (D) showing expression levels of indicated total proteins and their phosphorylated forms in integrin α2-deficient and control U343MG cells treated as indicated, n = 4–6. (E) Analysis of active ATF1 in U343MG cells upon integrin α2-knockdown and in controls with/without 6 Gy X-rays + TMZ. Western blots demonstrate ITGA2 siRNA knockdown efficiency, n = 3. Representative Western blots (F) and densitometry analysis (G) for ATF1-deficient and control U343MG cells treated as indicated demonstrating phospho-ERK1/2 (Thr202/Tyr204) and ERK1/2 expression levels, n = 3. (H) Analysis of ERK1 and ERK2 expression changes in integrin α2/ATF1-deficient cells 24 h after irradiation (IR) + temozolomide (TMZ), n = 5–6. All data are presented as mean ± SEM. Statistics were performed with unpaired two-tailed Student’s t-test. Multiple comparisons were performed using one-way ANOVA followed by Sidak test, *P < .05, **P < .01, ***P < .001, ****P < .0001.
Integrin α2-Knockdown Regulates ERK1 Protein Levels in GBM Cells in an ATF1-Dependent Manner
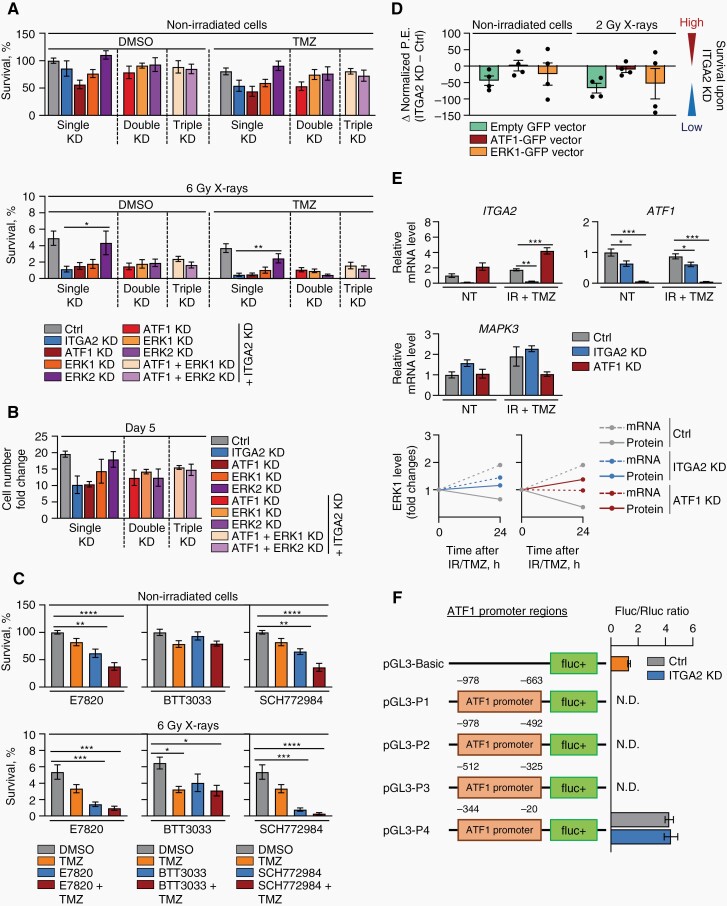
We addressed whether ATF1 is functionally downstream of integrin α2 but upstream of ERK1 and ERK2 by combinatorial knockdowns and measurement of clonogenic survival. We conducted single, double, and triple depletion of integrin α2, ATF1, ERK1, and ERK2 in GBM cells and analyzed colony formation and proliferation. Our data showed that in irradiated cells, ATF1 and ERK1 (not ERK2) single knockdown, double depletion of integrin α2 together with ATF1, ERK1, or ERK2 as well as triple knockdown of integrin α2 combined with ATF1/ERK1 or ATF1/ERK2 mediate radio- and radiochemosensitization similar to integrin α2 single knockdown (Figure 6A, B, Supplementary Table 3, Supplementary Figure 5B, C). Therefore, we deduce ATF1 and ERK1 to lie in the same signaling pathway downstream of integrin α2. To further confirm the general radio(chemo)sensitizing potential of integrin α2-targeting with and without ERK-targeting, we applied pharmacological integrin α2 (E7820, BTT3033) and ERK (SCH772984) inhibitors. While E7820 and SCH772984 showed already basal cytotoxic and chemosensitizing effects, all 3 agents proved the most efficient when administered in combination with irradiation (Figure 6C, Supplementary Figure 5D, E). From these data, we conclude that integrin α2, ATF1, and ERK1 unfold their essential roles in GBM cell survival only upon administration of irradiation and/or TMZ and their pharmacological targeting might have a therapeutic value in GBM treatment. In addition, we show that overexpression of ATF1 (not ERK1) almost completely abrogates the radiosensitization induced by integrin α2-depletion (Figure 6D, Supplementary Figures 5F and 6).
Figure 6.
Integrin α2-deficiency regulates the radiochemosensitivity of GBM cells via the ATF1/ERK1 axis. (A) Analysis of the clonogenic survival in U343MG cells transfected with control, ITGA2, ATF1, MAPK3 (targeting ERK1) or MAPK1 (targeting ERK2) siRNAs (and in combinations) upon indicated treatments, n = 4. (B) Growth rate of U343MG cells upon integrin α2 (ITGA2), ATF1, ERK1, or ERK2 knockdowns on day 5 after seeding, n = 3. (C) Analysis of radio(chemo)sensitizing effects of integrin α2 (E7820 and BTT3033) and ERK (SCH772984) inhibitors on U343MG cells, n = 4. (D) Differences in radiosensitizing efficiency in integrin α2-depleted U343MG cells transfected with either pTagGFP2-N-hATF1 or pTagGFP2-N-hERK1 vectors (empty pTagGFP2-N vector was used as a control) shown as Δ values of normalized plating efficiency (P.E.), n = 4. (E) Graph illustrating ITGA2, ATF1 and MAPK3 (=ERK1) mRNA levels in U343MG cells (upper panel) and mRNA-protein kinetics (lower panel) upon silencing of integrin α2 or ATF1, n = 3–6. (F) The indicated reporter constructs were transfected into integrin α2-deficient and control U343MG cells. Firefly in relation to Renilla luciferase levels were calculated. The empty vector pGL3-Basic was used as a reference, n = 3. N.D.— no signal detected. All data are shown as mean ± SEM. Statistical analysis was performed using one-way ANOVA followed by Sidak (A, B) or Dunnett (C, E) post hoc tests, *P < .05, **P < .01, ***P < .001, ****P < .0001.
To further untangle the mechanisms underlying radiochemosensitization upon integrin α2-targeting and its accompanying decline of ATF1 and induction of ERK1, we analyzed ATF1 and MAPK3 (=ERK1) mRNA expressions. In line with protein levels, integrin α2-deficiency led to reduced ATF1 mRNA levels compared to controls. However, in contrast to controls, MAPK3 (=ERK1) mRNA and protein levels are both increased in integrin α2- and ATF1-deficient cells (Figure 6E). Given reduced ATF1 mRNA expression upon integrin α2-knockdown, we next carried out a luciferase reporter assay to analyze ATF1 promoter activity. We observed that deletion of the region from −344 to −20 bp abrogates ATF1 promoter activity, while no difference in promoter activity was detectable between integrin α2-deficient and control cells (Figure 6F).
Discussion
Intrinsic and acquired resistance of cancer cells to therapy represents a major challenge in cancer treatment. Both types of resistance are associated with genetic, epigenetic, and microenvironmental changes.6,8 Therefore, understanding resistance mechanisms is of paramount clinical importance to significantly improve the existing therapeutic avenues. In the current research on GBM, we identified that (1) depletion of the adhesion receptor integrin α2 promotes radio(chemo)sensitization, (2) integrin α2-knockdown impairs proliferation, adhesion, and spreading, (3) integrin α2-silencing in combination with radio- and chemotherapy delays tumor growth and promotes mouse survival in an orthotopic GBM model, and, (4) the ATF1/ERK1 axis contributes to the radiochemosensitizing effect of integrin α2-knockdown.
Several studies highlighted the role of ECM-adhesion receptor interactions of cancer cells for the development of therapy resistance.7,8,24,29 Moreover, there is increasing knowledge and awareness of mutual and cooperative survival-advantaging interrelations between cell adhesion molecules and growth factor/cytokine receptors coalescing at focal adhesions.30 Therefore, we performed single and simultaneous siRNA-mediated silencing of integrin subunits and growth factor/cytokine receptors and identified integrin α2 as the most potent regulator of radio(chemo)resistance in GBM cells.
Previously, it was shown that integrin α2 is overexpressed in human malignancies in comparison to normal tissues and contributes to the regulation of self-renewal, apoptosis, cell cycle, and differentiation status of cancer cells.9–13 In the context of GBM and in line with our findings, Guo and coworkers demonstrated that integrin α2 blockade resulted in impaired migration of GBM cells.19 In our study, we confirmed that integrin α2-knockdown is essential for the spreading of GBM cells affecting their adhesive and invasive properties. In addition, we showed that integrin α2-deficiency causes antimitogenic effects in GBM cells while being dispensable for DNA damage repair. In contrast to other studies focused on understanding the effects of single integrin α2-knockdown on tumor cell survival, we investigated the role of integrin α2-depletion in GBM cells exposed to the clinical standard of a care treatment regimen consisting of X-ray irradiation and TMZ. We report that integrin α2-knockdown has a significant radio(chemo)sensitizing effect in most GBM cell models in vitro, which also translates into a prolongation of the survival of mice in an orthotropic GBM model. Excitingly, pharmacological inhibitors, i.e., E7820 and BTT3033, targeting integrin α2 provide confirmatory data on the radio(chemo)sensitizing potential integrin α2-blocking in GBM models.
In further investigations, we aimed to unravel the molecular mechanism underlying GBM cell radiochemosensitivity upon integrin α2-knockdown. High-throughput kinome profiling identified an overall reduction in STK kinase activity with only a marginal effect on the PTK profile in integrin α2-deficient, X-rays/TMZ-treated GBM cells. The majority of altered serine/threonine kinases turned out to belong to the MAPK signaling pathway. These are known to be dysregulated in GBM and associated with resistance to radio- and chemotherapy.31 The kinases ERK1, ERK2, and p38 represent key components of the MAPK cascade.32 Several previous studies demonstrated that specific residues within the cytoplasmic tail of integrin α2 participate in the regulation of ERK/p38 phosphorylation, which affects cell migration and cell cycle progression.33,34 Our kinome dataset also predicted a reduction in Akt kinase activity. Although Akt fuels into a large network separate from MAPK kinases, intensive crosstalk between Akt and MAPK signaling pathways have been discussed in numerous reports.35 Thus, in further experiments, we were unable to observe changes in phosphorylation of ERK1, ERK2, p38, and Akt in integrin α2-deficient GBM cells.16,33,34 However, we show that integrin α2-knockdown resulted in sustained protein expression of ERK1 after X-rays/TMZ treatment as compared to controls. These observations further support, despite their high level of structural identity, ERK1, and ERK2 to serve in a highly context-dependent manner as reviewed and discussed elsewhere.36 Genetic and pharmacological inhibition of ERK1 elicits radio(chemo)sensitization of GBM cells, however, ERK1-overexpression did not result in radioresistance. Thus, when ERK1 protein expression is considered after radiochemotherapy, both integrin α2- and ATF1-knockdowns led to similar stabilized ERK1 levels compared to controls. In the context of the presented survival data, this does not appear to have a detectable attenuating effect on radiosensitization by either integrin α2- or ATF1-silencing.
We continued our investigations of ERK1/2 and Akt signaling cascades by analyzing the important downstream effectors CREB/ATF1 and 4E-BP1 implicated in the transcription process and protein synthesis, respectively. Integrin α2-deficient GBM cells revealed a moderate reduction in 4E-BP1 phosphorylation, which was paralleled by a 2-fold decrease in ATF1 protein levels. ATF1 represents a pro-survival transcription factor critically functioning in the regulation of various physiological processes e.g. proliferation, survival, differentiation, and cell cycling.28 Till date, it is known that phosphorylation and transcriptional activity of ATF1 is regulated by components of the MAPK signaling cascades such as p38, mitogen- and stress-activated protein kinase (MSK) 1 and MSK2.37–39 Here, we first demonstrate ATF1 activity, mRNA and protein but not phosphorylation levels to be apparently controlled by integrin α2. Moreover, the reduction in ATF1 mRNA expression after silencing of integrin α2 was not associated with any expected changes in ATF1 promoter activity, suggesting possible posttranscriptional mRNA modifications (e.g., microRNAs, RNA methylation). Intriguing for the hierarchical positioning within the integrin α2-driven signal transduction network is the fact that reduction in the ATF1 protein level precedes changes in ERK1. Taken together, we show that integrin α2, ATF1, and ERK1 belong to the same signaling axis and regulate radiochemoresistance in GBM cells, the following aspects deserve further attention: (1) how integrin α2 mechanistically executes ATF1 mRNA expression;(2) how integrin α2 modulates compensatory induction of ERK1 in response to radiochemotherapy, and (3) whether this molecular circuitry is part of those bypass mechanisms in GBM which foster resistance and invasiveness, and (4) what distinguishes the nonresponder from the responder GBM cell models molecularly for therapeutic exploitation.
In summary, our study demonstrates that the loss of integrin α2 results in reduced proliferation, invasion, and adhesion of GBM cells. With high translational potency, we weigh our complementary in vitro and in vivo data demonstrating that integrin α2 targeting overcomes the radio(chemo)resistance of GBM cells, contributing to prolonged survival. In conclusion, integrin α2 emerges as an attractive therapeutic target for GBM when combined with the current standard therapy consisting of radiotherapy and TMZ.
Supplementary Material
Acknowledgments
We are grateful to K. Lamzus (University Medical Center Hamburg-Eppendorf, Hamburg, Germany) for providing GS-8 cells and L. Kunz-Schughart (University Hospital Dresden, Germany) for U251MG and LN405 cell lines. We thank I. Lange, C. Krause and L. Stolz-Kieslich for their technical assistance. We are grateful to K. Rӧbel for the support with retroviral transduction.
Contributor Information
Irina Korovina, OncoRay—National Center for Radiation Research in Oncology, Faculty of Medicine Carl Gustav Carus, Technische Universität Dresden, Dresden, Germany; Helmholtz-Zentrum Dresden - Rossendorf, Institute of Radiooncology–OncoRay, Dresden, Germany.
Anne Vehlow, OncoRay—National Center for Radiation Research in Oncology, Faculty of Medicine Carl Gustav Carus, Technische Universität Dresden, Dresden, Germany; National Center for Tumor Diseases (NCT), Partner Site Dresden, German Cancer Research Center (DKFZ), Heidelberg, Germany.
Achim Temme, German Cancer Consortium (DKTK), Partner Site Dresden, and German Cancer Research Center (DKFZ), Heidelberg, Germany; National Center for Tumor Diseases (NCT), Partner Site Dresden, German Cancer Research Center (DKFZ), Heidelberg, Germany; Department of Neurosurgery, Section Experimental Neurosurgery and Tumor Immunology, University Hospital Carl Gustav Carus, Technische Universität Dresden, Dresden, Germany.
Nils Cordes, OncoRay—National Center for Radiation Research in Oncology, Faculty of Medicine Carl Gustav Carus, Technische Universität Dresden, Dresden, Germany; Helmholtz-Zentrum Dresden - Rossendorf, Institute of Radiooncology–OncoRay, Dresden, Germany; German Cancer Consortium (DKTK), Partner Site Dresden, and German Cancer Research Center (DKFZ), Heidelberg, Germany; Department of Radiotherapy and Radiation Oncology, University Hospital Carl Gustav Carus, Technische Universität Dresden, Dresden, Germany; National Center for Tumor Diseases (NCT), Partner Site Dresden, German Cancer Research Center (DKFZ), Heidelberg, Germany.
Funding
Funding for this work was provided by the Wilhelm Sander Foundation (2017.072.1 to N.C.). and the EFRE Europäischer Fonds für regionale Entwicklung, Europa, fördert Sachsen (100066308 to N.C.).
Conflict of Interests:
The authors declare no conflict of interest.
Authorship
Performance of the experiments: I.K. Conception and design: I.K., A.V., and N.C. Data analysis and interpretation: I.K., A.V., and N.C. Design and performance of animal research: I.K. and A.V. Provided materials: A.T. Study supervision: N.C. Manuscript writing: I.K., A.V., and N.C. Manuscript editing and final approval: all authors.
References
- 1. Louis DN, Perry A, Reifenberger G, et al. The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 2. Becker KP, Yu J. Status quo-standard-of-care medical and radiation therapy for glioblastoma. Cancer J. 2012;18(1):12–19. [DOI] [PubMed] [Google Scholar]
- 3. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 4. Weller M, Wick W, Aldape K, et al. Glioma. Nat Rev Dis Prim. 2015;1:15017. doi: 10.1038/nrdp.2015.17 [DOI] [PubMed] [Google Scholar]
- 5. Ramirez YP, Weatherbee JL, Wheelhouse RT, Ross AH. Glioblastoma multiforme therapy and mechanisms of resistance. Pharmaceuticals (Basel). 2013;6(12):1475–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Varn FS, Johnson KC, Martinek J, et al. Glioma progression is shaped by genetic evolution and microenvironment interactions. Cell. 2022;185(12):2184–2199.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cooper J, Giancotti FG. Integrin signaling in cancer: mechanotransduction, stemness, epithelial plasticity, and therapeutic resistance. Cancer Cell. 2019;35(3):347–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Deville SS, Cordes N. The extracellular, cellular, and nuclear stiffness, a trinity in the cancer resistome—a review. Front Oncol. 2019;9:1376. doi: 10.3389/fonc.2019.01376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Badea L, Herlea V, Dima SO, Dumitrascu T, Popescu I. Combined gene expression analysis of whole-tissue and microdissected pancreatic ductal adenocarcinoma identifies genes specifically overexpressed in tumor epithelia. Hepatogastroenterology. 2008;55(88):2016–2027. [PubMed] [Google Scholar]
- 10. Cui J, Chen Y, Chou W-C, et al. An integrated transcriptomic and computational analysis for biomarker identification in gastric cancer. Nucleic Acids Res. 2011;39(4):1197–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lian X-Y, Zhang W, Wu D-H, et al. Methylation-independent ITGA2 overexpression is associated with poor prognosis in de novo acute myeloid leukemia. J Cell Physiol. 2018;233(12):9584–9593. [DOI] [PubMed] [Google Scholar]
- 12. Estilo CL, O-Charoenrat P, Talbot S, et al. Oral tongue cancer gene expression profiling: identification of novel potential prognosticators by oligonucleotide microarray analysis. BMC Cancer. 2009;9:11. doi: 10.1186/1471-2407-9-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang Q, Bavi P, Wang JY, Roehrl MH. Immuno-proteomic discovery of tumor tissue autoantigens identifies olfactomedin 4, CD11b, and integrin alpha-2 as markers of colorectal cancer with liver metastases. J Proteomics. 2017;168:53–65. doi: 10.1016/j.jprot.2017.06.021 [DOI] [PubMed] [Google Scholar]
- 14. Wong KF, Liu AM, Hong W, Xu Z, Luk JM. Integrin α2β1 inhibits MST1 kinase phosphorylation and activates Yes-associated protein oncogenic signaling in hepatocellular carcinoma. Oncotarget. 2016;7(47):77683–77695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ren D, Zhao J, Sun Y, et al. Overexpressed ITGA2 promotes malignant tumor aggression by up-regulating PD-L1 expression through the activation of the STAT3 signaling pathway. J Exp Clin Cancer Res. 2019;38(1):485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ma L, Sun Y, Li D, et al. Overexpressed ITGA2 contributes to paclitaxel resistance by ovarian cancer cells through the activation of the AKT/ FoxO1 pathway. Aging (Albany NY). 2020;12(6):5336–5351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chuang YC, Wu HY, Lin YL, et al. Blockade of ITGA2 induces apoptosis and inhibits cell migration in gastric cancer. Biol Proced Online. 2018:20:10. doi: 10.1186/s12575-018-0073-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang Q, Cao T, Guo K, et al. Regulation of integrin subunit alpha 2 by miR-135b-5p modulates chemoresistance in gastric cancer. Front Oncol. 2020;10:308. doi: 10.3389/fonc.2020.00308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guo P, Moses-Gardner A, Huang J, Smith ER, Moses MA. ITGA2 as a potential nanotherapeutic target for glioblastoma. Sci Rep. 2019;9(1):6195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vehlow A, Klapproth E, Storch K, et al. Adhesion- and stress-related adaptation of glioma radiochemoresistance is circumvented by β1 integrin/JNK co-targeting. Oncotarget. 2017;8(30):49224–49237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Deville SS, Silva LFD, Vehlow A, Cordes N. C-Abl tyrosine kinase is regulated downstream of the cytoskeletal protein synemin in head and neck squamous cell carcinoma radioresistance and DNA repair. Int J Mol Sci . 2020;21(19):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tang Z, Li C, Kang B, et al. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45(W1):W98–W102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Scully R, Panday A, Elango R, Willis NA. DNA double-strand break repair-pathway choice in somatic mammalian cells. Nat Rev Mol Cell Biol. 2019;20(11):698–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vehlow A, Klapproth E, Jin S, et al. Interaction of Discoidin Domain Receptor 1 with a 14-3-3-Beclin-1-Akt1 complex modulates glioblastoma therapy sensitivity. Cell Rep. 2019;26(13):3672–3683.e7. [DOI] [PubMed] [Google Scholar]
- 25. Pearce LR, Komander D, Alessi DR. The nuts and bolts of AGC protein kinases. Nat Rev Mol Cell Biol. 2010;11(1):9–22. [DOI] [PubMed] [Google Scholar]
- 26. She Q-B, Halilovic E, Ye Q, et al. 4E-BP1 is a key effector of the oncogenic activation of the AKT and ERK signaling pathways that integrates their function in tumors. Cancer Cell. 2010;18(1):39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Uhlén M, Björling E, Agaton C, et al. A human protein atlas for normal and cancer tissues based on antibody proteomics. Mol Cell Proteomics. 2005;4(12):1920–1932. [DOI] [PubMed] [Google Scholar]
- 28. Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35(4):605–623. [DOI] [PubMed] [Google Scholar]
- 29. Dickreuter E, Eke I, Krause M, et al. Targeting of β1 integrins impairs DNA repair for radiosensitization of head and neck cancer cells. Oncogene. 2016;35(11):1353–1362. [DOI] [PubMed] [Google Scholar]
- 30. Hamidi H, Ivaska J. Every step of the way: integrins in cancer progression and metastasis. Nat Rev Cancer. 2018;18(9):533–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pearson JRD, Regad T. Targeting cellular pathways in glioblastoma multiforme. Signal Transduct Target Ther. 2017;2(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26(22):3279–3290. [DOI] [PubMed] [Google Scholar]
- 33. Klekotka PA, Santoro SA, Wang H, Zutter MM. Specific residues within the α2 integrin subunit cytoplasmic domain regulate migration and cell cycle progression via distinct MAPK pathways. J Biol Chem. 2001;276(34):32353–32361. [DOI] [PubMed] [Google Scholar]
- 34. Klekotka PA, Santoro SA, Zutter MM. α2 Integrin subunit cytoplamic domain-dependent cellular migration requires p38 MAPK. J Biol Chem. 2001;276(12):9503–9511. [DOI] [PubMed] [Google Scholar]
- 35. Cao Z, Liao Q, Su M, et al. AKT and ERK dual inhibitors: the way forward? Cancer Lett. 2019;459:30–40. doi: 10.1016/j.canlet.2019.05.025 [DOI] [PubMed] [Google Scholar]
- 36. Buscà R, Pouysségur J, Lenormand P. ERK1 and ERK2 map kinases: specific roles or functional redundancy? Front Cell Dev Biol. 2016;4:1–23. doi: 10.3389/fcell.2016.00053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gupta P, Prywes R. ATF1 phosphorylation by the ERK MAPK Pathway is required for epidermal growth factor-induced c-jun expression. J Biol Chem. 2002;277(52):50550–50556. [DOI] [PubMed] [Google Scholar]
- 38. Wiggin GR, Soloaga A, Foster JM, et al. MSK1 and MSK2 are required for the mitogen- and stress-induced phosphorylation of CREB and ATF1 in fibroblasts. Mol Cell Biol. 2002;22(8):2871–2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Al-Huseini LMA, Aw Yeang HX, Hamdam JM, et al. Heme oxygenase-1 regulates dendritic cell function through modulation of p38 MAPK-CREB/ATF1 signaling. J Biol Chem. 2014;289(23):16442–16451. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.