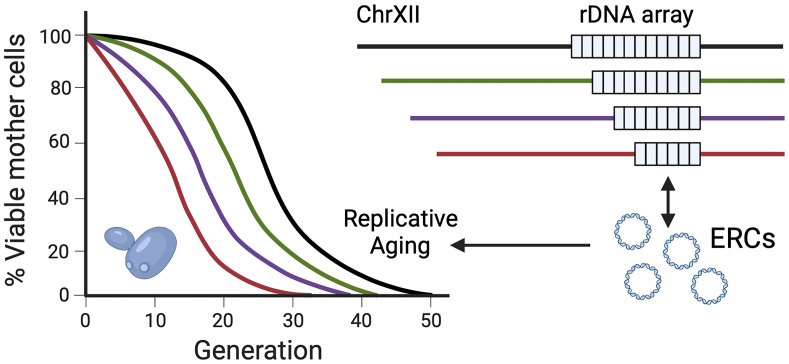
The budding yeast, Saccharomyces cerevisiae, is a popular single-cell eukaryotic model for studying mechanisms of aging, having played key roles in the identification and characterization of several conserved longevity factors, including the canonical NAD+-dependent histone deacetylase Sir2 that silences transcription and maintains stability of the ribosomal DNA (rDNA) tandem array on chromosome XII (reviewed in ref. 1). Other Sir2 protein family members, the sirtuins, regulate numerous cellular functions linked with the aging of invertebrate models and mammals, thus making them central players in current aging research (2). The rDNA tandem arrays are among the most unstable loci in eukaryotic genomes, comprising up to hundreds of rRNA genes transcribed by RNA polymerase I in the nucleolus to drive ribosome biogenesis. In S. cerevisiae, the single rDNA array is especially unstable, as mother cells replicatively age due to DNA double-strand breaks (DSBs) within the intergenic spacers. Repair of these breaks can result in the excision and accumulation of extrachromosomal rDNA circles (ERCs) in mother cells that ultimately contribute to their senescence (3). Silencing by Sir2 normally suppresses improper excision in young cells, but natural depletion of Sir2 in older cells causes instability (4). In PNAS, Hotz et al. (5) now demonstrate that a major determinant of ERC and Sir2 levels, and therefore replicative aging in most strains, is the actual length (copy number) of the rDNA tandem array (Fig. 1). This has important implications for the interpretation of yeast replicative lifespan (RLS) studies and signals that rDNA copy number should be addressed in metazoan aging studies.
Fig. 1.
S. cerevisiae RLS is dictated by the length of the rDNA tandem array located on chromosome XII. As the array becomes shorter, more ERCs accumulate in the aging mother cells, contributing to their senescence. ERCs can reintegrate into the short array while it is destabilized in order to expand again, a process regulated by Sir2 and the RNA polymerase I transcription factor, UAF. Created with BioRender.
RLS of S. cerevisiae is defined as the number of times a mother cell divides (buds) before losing viability. This number is traditionally measured by manual microscopic dissection of mother and daughter cells on the surface of an agar plate, and, more recently, using high-throughput microfluidics devices that automatically track hundreds of mother cells, as carried out by Hotz et al. (5). RLS is considered an aging model for dividing cells of multicellular organisms, such as stem cells, which depend on growth-related functions like nutrient signaling, translation, and DNA replication/repair, all of which can lead to genome instability with age.
Accordingly, genomic instability is one of the hallmarks of aging conserved from yeast to mammals (6). DSBs are a severe form of DNA damage that result in cell death if left unrepaired. The rDNA array is especially prone to DSBs because of the inherent difficulty of DNA replication through a highly transcribed repetitive locus and the unusual structures associated with it, such as R-loops or G-quadruplexes (7). Replication fork stalling at such obstacles during replication stress can result in DSBs due to fork collapse (8). S. cerevisiae rDNA also harbors a major replication fork block (RFB) site within the intergenic spacer mediated by a site-specific DNA binding protein called Fob1 (9). This RFB is unidirectional, allowing replication to proceed in the same direction as rRNA transcription, while preventing replication forks moving in the opposite direction from colliding with RNA Pol I machinery. When DSBs occur at the RFB, unequal sister chromatin exchange during homologous repair can result in repeat contraction and excision of ERCs, which then replicate and exponentially accumulate in mother cells at each S phase (3). They are also heavily transcribed by RNA Pol I to produce excessive precursor rRNA, which is proposed to disrupt nuclear homeostasis and increase cell mortality (10). Importantly, deleting FOB1 prevents the RFB and extends RLS (11), and is therefore commonly used in the field as a genetic tool to prevent rDNA recombination and stabilize the array.
ERC levels in young cells are anticorrelated with the length of the rDNA array (12), but, previously, there had not been clear functional evidence for the array length consistently impacting RLS (13, 14). To address this gap, Hotz et al. (5) generated a large number of isogenic “wild-type” (WT) laboratory strains with varying rDNA array sizes, either by random meiotic recombination through sporulation and tetrad dissection or by direct replacement of random-sized rDNA segments by transformation with a hygromycin-selectable PCR cassette. Array lengths were determined by whole-genome sequencing (WGS) and contour-clamped homogeneous electric-field electrophoresis gel analysis, yielding strains with rDNA copy numbers ranging from ∼50 to 250. RLS was quantified for each strain using a microfluidics device, revealing a remarkable positive correlation between lifespan and array sizes up to 150 copies. No further lifespan improvement was observed for strains with even longer arrays. Furthermore, based on the WGS results, ∼70% of the RLS variation between strains could be accounted for by rDNA differences, consistent with earlier unbiased quantitative trait locus (QTL) analyses that identified polymorphisms in the rDNA origin of replication (rARS) as major variants associated with lifespan (15, 16).
To determine why ERC levels anticorrelate with chromosomal rDNA copy number, Hotz et al. (5) took advantage of their strain collection with varying rDNA array sizes to measure ERC levels in replicatively aged mother cell populations isolated using a modified miniature-chemostat aging device (17). Previous analyses comparing ERCs and copy number were performed with asynchronous log-phase cultures (12). As predicted, ERCs declined in aged cells as the chromosomal rDNA copy number increased, but, again, no additional effect was observed once the array exceeded 150 copies. Deleting FOB1 erased this inverse relationship, resulting in low ERCs and extended RLS regardless of copy number. Together, these results suggest the effect of rDNA array length on RLS works by dictating ERC levels. It should be noted that the Kobayashi group previously identified a specific mutation in the rDNA replication origin (rARSΔ-3amp) that prevented ERC accumulation yet destabilized the rDNA and shortened RLS in a FOB1-dependent manner (18). They concluded that instability of the rDNA array, not ERC accumulation, was the primary RLS effector. This distinction returns us to the rARS acting as a major QTL for RLS. A polymorphism that extended RLS had reduced replication origin firing at the rARS, and also caused improved origin activity at other ARS elements across the genome, presumably by releasing limiting replication factors from the rDNA to other loci (15). Reduced rARS activity was also proposed as a mechanism by which caloric restriction, glucose restriction in the yeast system, extends RLS. We can therefore speculate that changes in rDNA copy number could impact origin activity, which then affects ERC accumulation.
All of these related mechanisms are dependent on Sir2, the histone deacetylase targeted to the RFB by Fob1, where it represses noncoding RNA transcription by an intergenic RNA Pol II promoter called E-pro (19). Transcription from E-pro displaces cohesin, causing local misalignment of sister chromatids and ERC formation (19). Sir2 represses E-pro to keep the rDNA repeats properly aligned, thus preventing unequal sister chromatid exchange. S. cerevisiae also has an elegant mechanism for tying Sir2 expression to maintenance of rDNA copy number. The Shore laboratory first showed that reduced rDNA copy number causes reduced Sir2 protein levels (14), followed by Iida and Kobayashi (20), who elucidated a model whereby the limiting RNA Pol I transcription factor UAF redistributes from the rDNA to the SIR2 promoter when the array size contracts. UAF then represses SIR2 transcription, explaining the reduced Sir2 protein observed earlier. Reduction of Sir2 protein derepresses E-pro and destabilizes the short rDNA array, allowing ERCs to recombine back into the array to rebuild it (12). As the rDNA copy number increases and recaptures UAF binding, SIR2 expression increases, and the longer rDNA array is restabilized (20). Hotz et al. (5) take this rDNA copy number counting model a step further and show increased chromatin accessibility at the UAF binding site upstream of SIR2 as rDNA copy number decreases, most likely due to UAF reorganizing the structure. UAF consists of several protein subunits, including histones H3 and H4 (21), so it could potentially wrap DNA and locally substitute for a nucleosome at the SIR2 promoter. Taken together, the results from Hotz et al. integrate well with other published works to yield a unifying model centered on the rDNA array size as a driving factor of replicative aging.
From a practical standpoint of laboratories performing yeast RLS experiments, the significant impact of rDNA copy number on lifespan carries some salient implications. First, variants in rDNA copy number are common passenger mutations introduced by the standard lithium acetate-mediated transformation protocol and are also common in the popular yeast knock-out collection strains (13, 22). Second, Hotz et al. (5) revisit several deletion mutants already reported to extend RLS, ascertaining whether they were influenced by introduced rDNA copy number variations. RLS of each tested mutant was dramatically altered by copy number, similar to the effect in WT strains. However, when normalized for copy number, three of the seven mutants no longer extended RLS, suggesting the original lifespan extension phenotypes could have been due to rDNA copy number differences. Moving forward, rDNA copy number should clearly be monitored for strains used in RLS experiments.
Aging studies in yeast are most impactful when informing about mechanisms of aging in more-complex eukaryotes. In this case, surprisingly, very little is known about the role that rDNA array instability, ERCs, or copy number play in the aging process for the common model organisms used for aging research. Given the conservation of rDNA array organization and DNA repair systems from yeast to humans, the pioneering work dissecting mechanisms of rDNA instability and functional links to aging in yeast will provide a detailed roadmap for future, and necessary, analyses in multicellular organisms.
Acknowledgments
I thank Lindsey Power and Elisa Enriquez Hesles for critically reading the manuscript. Research in the J.S.S. lab is supported by NIH Grants GM075240, GM127394, and CA241905.
Footnotes
The author declares no competing interest.
See companion article, “rDNA array length is a major determinant of replicative lifespan in budding yeast,” 10.1073/pnas.2119593119.
References
- 1.Wierman M. B., Smith J. S., Yeast sirtuins and the regulation of aging. FEMS Yeast Res. 14, 73–88 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Houtkooper R. H., Pirinen E., Auwerx J., Sirtuins as regulators of metabolism and healthspan. Nat. Rev. Mol. Cell Biol. 13, 225–238 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sinclair D. A., Guarente L., Extrachromosomal rDNA circles—A cause of aging in yeast. Cell 91, 1033–1042 (1997). [DOI] [PubMed] [Google Scholar]
- 4.Lindstrom D. L., Leverich C. K., Henderson K. A., Gottschling D. E., Replicative age induces mitotic recombination in the ribosomal RNA gene cluster of Saccharomyces cerevisiae. PLoS Genet. 7, e1002015 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hotz M., et al. , rDNA array length is a major determinant of replicative lifespan in budding yeast. Proc. Natl. Acad. Sci. U.S.A. 119, e2119593119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.López-Otín C., Blasco M. A., Partridge L., Serrano M., Kroemer G., The hallmarks of aging. Cell 153, 1194–1217 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Datta A., Pollock K. J., Kormuth K. A., Brosh R. M. Jr., G-quadruplex assembly by ribosomal DNA: Emerging roles in disease pathogenesis and cancer biology. Cytogenet. Genome Res. 161, 285–296 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeman M. K., Cimprich K. A., Causes and consequences of replication stress. Nat. Cell Biol. 16, 2–9 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kobayashi T., Horiuchi T., A yeast gene product, Fob1 protein, required for both replication fork blocking and recombinational hotspot activities. Genes Cells 1, 465–474 (1996). [DOI] [PubMed] [Google Scholar]
- 10.Morlot S., et al. , Excessive rDNA transcription drives the disruption in nuclear homeostasis during entry into senescence in budding yeast. Cell Rep. 28, 408–422.e4 (2019). [DOI] [PubMed] [Google Scholar]
- 11.Defossez P. A., et al. , Elimination of replication block protein Fob1 extends the life span of yeast mother cells. Mol. Cell 3, 447–455 (1999). [DOI] [PubMed] [Google Scholar]
- 12.Mansisidor A., et al. , Genomic copy-number loss is rescued by self-limiting production of DNA circles. Mol. Cell 72, 583–593.e4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwan E. X., Wang X. S., Amemiya H. M., Brewer B. J., Raghuraman M. K., rDNA copy number variants are frequent passenger mutations in Saccharomyces cerevisiae deletion collections and de novo transformants. G3 (Bethesda) 6, 2829–2838 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michel A. H., Kornmann B., Dubrana K., Shore D., Spontaneous rDNA copy number variation modulates Sir2 levels and epigenetic gene silencing. Genes Dev. 19, 1199–1210 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwan E. X., et al. , A natural polymorphism in rDNA replication origins links origin activation with calorie restriction and lifespan. PLoS Genet. 9, e1003329 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stumpferl S. W., et al. , Natural genetic variation in yeast longevity. Genome Res. 22, 1963–1973 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hendrickson D. G., et al. , A new experimental platform facilitates assessment of the transcriptional and chromatin landscapes of aging yeast. eLife 7, e39911 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ganley A. R., Ide S., Saka K., Kobayashi T., The effect of replication initiation on gene amplification in the rDNA and its relationship to aging. Mol. Cell 35, 683–693 (2009). [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi T., Ganley A. R., Recombination regulation by transcription-induced cohesin dissociation in rDNA repeats. Science 309, 1581–1584 (2005). [DOI] [PubMed] [Google Scholar]
- 20.Iida T., Kobayashi T., RNA polymerase I activators count and adjust ribosomal RNA gene copy number. Mol. Cell 73, 645–654.e13 (2019). [DOI] [PubMed] [Google Scholar]
- 21.Keener J., Dodd J. A., Lalo D., Nomura M., Histones H3 and H4 are components of upstream activation factor required for the high-level transcription of yeast rDNA by RNA polymerase I. Proc. Natl. Acad. Sci. U.S.A. 94, 13458–13462 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saka K., Takahashi A., Sasaki M., Kobayashi T., More than 10% of yeast genes are related to genome stability and influence cellular senescence via rDNA maintenance. Nucleic Acids Res. 44, 4211–4221 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]