Abstract
Introduction
Trophoblast cell surface antigen 2 (TROP2) is the target of sacituzumab govitecan, an antibody-drug conjugate approved for treatment of triple negative breast cancer and urothelial carcinoma.
Methods
A tissue microarray containing 18,563 samples from 150 different tumor types and subtypes as well as 608 samples of 76 different normal tissue types was analyzed by TROP2 immunohistochemistry.
Results
TROP2 positivity was found in 109 tumor categories, including squamous cell carcinomas of various origins, urothelial, breast, prostate, pancreatic, and ovarian cancers (>95% positive). High TROP2 expression was linked to advanced stage (p = 0.0069) and nodal metastasis (p < 0.0001) in colorectal cancer as well as to nodal metastasis in gastric adenocarcinoma (p = 0.0246) and papillary thyroid cancer (p = 0.0013). Low TROP2 expression was linked to advanced stage in urothelial carcinoma (p < 0.0001), high pT (p = 0.0024), and high grade (p < 0.0001) in breast cancer, as well as with high Fuhrmann grade (p < 0.0001) and pT stage (p = 0.0009) in papillary renal cell carcinomas.
Conclusion
TROP2 is expressed in many epithelial neoplasms. TROP2 deregulation can be associated with cancer progression in a tumor-type dependent manner. Since anti-TROP2 cancer drugs have demonstrated efficiency, they may be applicable to a broad range of tumor entities in the future.
Keywords: Trophoblast cell surface antigen 2, Tissue microarray, Immunohistochemistry, Neoplastic tissue, Epithelial neoplasm, Breast cancer, Urothelial carcinomas
Introduction
Trophoblast cell surface antigen 2 (TROP2), also known as tumor-associated calcium signal transducer 2 or EpCAM 2, is a membrane glycoprotein coded by the tumor-associated calcium signal transducer 2 gene at chromosome 1p32 [1, 2]. TROP2 acts as a cell surface receptor with a role in cell self-renewal, proliferation, and transformation [3, 4, 5]. In embryonic development, TROP2 plays a critical role in placenta formation, embryo implantation, stem cell proliferation, and organ development [5]. TROP2 can be found overexpressed in many cancer types. Consistent with its role in embryogenesis, TROP2 expression is regulated by several oncogenic transcription factors such as CREB1, nuclear factor-κB, HOXA10, HNF4A, TP63, TP53, ERG, HNF1A/TCF-1, and FOXP3 [6, 7]. TROP2 knockout cells show a disturbed proliferation, while overexpression of TROP2 accelerates the cancer cell cycle and drives cancer growth [8].
TROP2 is the target of sacituzumab govitecan (SG; TrodelvyTM), an antibody-drug conjugate that specifically targets TROP2 via the humanized anti-TROP2 antibody, hRS7 IgG which is conjugated to SN-38, the active metabolite of irinotecan [9]. SG has demonstrated significant clinical benefit in metastatic triple negative breast cancer [10], hormone-positive breast cancer [11], small-cell lung cancer [12], nonsmall-cell lung cancer [13], and metastatic urothelial carcinomas [14]. SG has recently been granted accelerated approval by the FDA for metastatic triple negative breast cancer patients that have failed at least two prior therapies [15]. SG has also been granted Fast Track Designation from the FDA for the treatment of adult urothelial cancer patients in the neoadjuvant/adjuvant, locally advanced or metastatic setting who have previously received a programmed death receptor-1 or programmed death-ligand 1 inhibitor and a platinum-containing chemotherapy or who are platinum ineligible and have previously received a programmed death receptor-1 or programmed death-ligand 1 inhibitor [16]. A number of studies have analyzed TROP2 expression in cell lines and clinical samples of urothelial [17], salivary gland [18], ovarian [19, 20, 21], endometrial [22], gastric [23, 24, 25], oral squamous cell [26, 27], lung [28], and cervical [29] carcinomas. However, the predictive role of TROP2 expression for response to SG is not entirely clear. Although high response rates have been reported particularly from cancers with moderate to strong TROP2 expression, there are also studies suggesting response in tumors with low TROP2 levels [12, 30, 31].
Currently, most available data on TROP2 expression in cancer tissues are based on RNA profiling and are available from public databases such as The Cancer Genome Atlas (TCGA) Research Network (https://www.cancer.gov.tcga) or the Gene Expression Omnibus (GEO) [32]. Only few studies have so far analyzed large cohorts of cancers for protein expression by immunohistochemistry (IHC) [33, 34]. To better understand the prevalence of TROP2 protein expression in cancer, TROP2 expression was analyzed in more than 18,000 tumor tissue samples from 150 different tumor types and subtypes as well as 76 non-neoplastic tissue categories by IHC in a tissue microarray (TMA) format in this study.
Material and Methods
Tissue Microarrays
The normal TMA was composed of 8 samples from 8 different donors for each of 76 different normal tissue types (608 samples on one slide). The cancer TMAs contained a total of 18,563 primary tumors from 150 tumor types and subtypes. Detailed histopathological data on grade, pathological tumor (pT) stage, or pathological lymph node (pN) status were available from subsets of breast cancers (n = 2,139), urothelial carcinomas (n = 1,073), high-grade serous ovarian cancers (n = 344), endometroid endometrial cancers (n = 160), colorectal (n = 2,351), gastric (n = 327), and pancreatic carcinomas (n = 598) as well as clear cell (n = 1,224) and papillary (n = 310) renal cell carcinomas. Clinical follow-up data were available from 877 breast cancer, 850 kidney cancer, and 254 bladder cancer patients (treated by cystectomy) with a median follow-up time of 43/39/14 months (range 1–88; 1–250; 1–77). The composition of both normal and cancer TMAs is described in detail in the result section. All samples were retrieved from the archives of the Institutes of Pathology, University Hospital of Hamburg, Germany; the Institute of Pathology, Clinical Center Osnabrueck, Germany; and Department of Pathology, Academic Hospital Fuerth, Germany. Tissues were fixed in 4% buffered formalin and then embedded in paraffin. The TMA manufacturing process has previously been described in detail [35, 36]. In brief, one tissue spot (diameter: 0.6 mm) per patient was transmitted from a cancer containing donor block into an empty recipient paraffin block. The use of archived remnants of diagnostic tissues for manufacturing of TMAs and their analysis for research purposes as well as patient data analysis has been approved by local laws (HmbKHG, §12) and by the Local Ethics Committee (Ethics Commission Hamburg, WF-049/09). All work has been carried out in compliance with the Helsinki Declaration.
Immunohistochemistry
Freshly prepared TMA sections were immunostained on 1 day in one experiment. Slides were deparaffinized with xylol, rehydrated through a graded alcohol series, and exposed to heat-induced antigen retrieval for 5 min in an autoclave at 121°C in pH 7.8 DakoTarget Retrieval SolutionTM (Agilent, Santa Clara, CA, USA; #S2367). Endogenous peroxidase activity was blocked with Dako Peroxidase-Blocking SolutionTM (Agilent, Santa Clara, CA, USA; #52023) for 10 min. Primary antibody-specific against TROP2 protein (recombinant rabbit monoclonal, MSVA-733R, MS Validated Antibodies, Hamburg, Germany) was applied at 37°C for 60 min at a dilution of 1:150. For the purpose of antibody validation, the normal tissue TMA was also analyzed with the polyclonal goat TROP2 antibody (R&D; # AF650) at a dilution of 1:450 and an otherwise identical protocol. Bound antibody was visualized using the EnVision KitTM (Agilent, Santa Clara, CA, USA; #K5007) according to the manufacturer's directions. The sections were counterstained with haemalaun. For tumor tissues, the percentage of TROP2-positive tumor cells was estimated and the staining intensity was semiquantitatively recorded (0, 1+, 2+, 3+). For statistical analyses, the staining results were categorized into four groups as follows: negative − no staining at all, weak staining − staining intensity of 1+ in ≤70% or staining intensity of 2+ in ≤30% of tumor cells, moderate staining − staining intensity of 1+ in >70%, staining intensity of 2+ in >30% but in ≤70%, or staining intensity of 3+ in ≤30% of tumor cells, and strong staining − staining intensity of 2+ in >70% or staining intensity of 3+ in >30% of tumor cells.
Statistics
Statistical calculations were performed with JMP 14 software (SAS Institute Inc., Cary, NC, USA). Contingency tables and the χ2 were performed to search for associations between TROP2 immunostaining and tumor phenotype. Survival curves were calculated according to Kaplan-Meier. The log-rank test was applied to detect significant differences between groups. A p value of ≤0.05 was defined as significant.
Results
Technical Aspects
A total of 16,024 (86.3%) of 18,563 tumor samples were interpretable in our TMA analysis. Noninterpretable samples demonstrated lack of unequivocal tumor cells or loss of the tissue spot during technical procedures. Enough samples of each normal tissue type were evaluable to enable a characterization of the normal tissue staining pattern.
TROP2 in Normal Tissues
TROP2 immunostaining was always membranous and found in many epithelial cell types. TROP2 staining was strong in all keratinizing and nonkeratinizing squamous epithelia. In the skin and the esophagus, the basal cell layers were either negative or markedly less stained, while all cell layers of the squamous epithelium of the uterine cervix and the tonsil surface showed an identical staining intensity. Skin appendices (sebaceous glands, hair follicles), tonsil crypt epithelium, and epithelial cells of the thymus including corpuscles of Hassall's also showed strong TROP2 staining. A strong TROP2 positivity was seen in all cells of the respiratory epithelium, while a moderate staining occurred in pneumocytes and in bronchial glands. In the placenta, staining was strong in amnion, chorion, and cytotrophoblast cells. TROP2 staining was largely absent in the gastrointestinal tract (GIT), with the exception of the superficial epithelial cell layers of the stomach and few scattered TROP2-positive epithelial cells (<0.1%) in the duodenum, ileum, appendix, and colon epithelium. In the pancreas, a strong positivity occurred in excretory and intercalated ducts, while acinar cells showed a variable staining, predominantly located at the apical membranes. Islets of Langerhans were TROP2 negative. A strong TROP2 staining was also seen in all epithelial cells of salivary glands, gallbladder epithelium, intrahepatic bile ducts, breast glands, fallopian tube (not all cells), endocervix, endometrium (not all glands of secretion phase), urothelium, prostate (less intensive staining in basal than in luminal cells), most epithelial cells of the seminal vesicle, and the epididymis. In the kidney, a strong TROP2 immunostaining occurred in distal tubuli and collecting ducts, while staining was weak to moderate in the parietal layer of the Bowman capsule. In the thyroid gland, a moderate to strong TROP2 staining of apical membranes was seen in a variable number of follicles. A small subset of epithelial cells of the adenohypophysis also showed a moderate TROP2 positivity. A small subset of positive cells was seen in the bone marrow, perhaps reflecting granulocyte precursor cells. Examples of TROP2 immunostaining in normal tissues are shown in Figure 1. TROP2 immunostaining was always absent in Brunner glands, hepatocytes, testis, decidua cells, ovary (stroma, follicular cysts, corpus luteum), adrenal gland, parathyroid gland, aorta, fat, muscles of all types, and the brain. All these findings were obtained by using the monoclonal rabbit recombinant antibody MSVA-733R and the goat polyclonal antibody AF650, although the polyclonal antibody resulted into somewhat more background staining (online suppl. Fig. 1; for all online suppl. material, see www.karger.com/doi/10.1159/000522206).
Fig. 1.
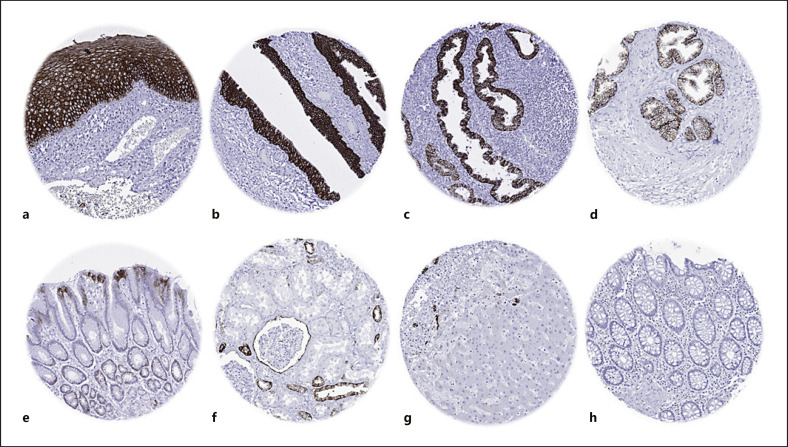
TROP2 immunostaining of normal tissues. The panels show a strong TROP2 positivity of surface epithelial cells of the tonsil (a), urothelium of the urinary bladder (b), and the endometrium (c) as well as in acinar and basal cells of the prostate (d). TROP2 staining is somewhat weaker and largely limited to the most apical elements of the surface epithelium in the stomach antrum (e), distal tubuli and the visceral layer of the Bowman capsule of the kidney (f), and intrahepatic bile ducts of the liver (g). TROP2 immunostaining is lacking in colon epithelial cells (h).
TROP2 in Cancer
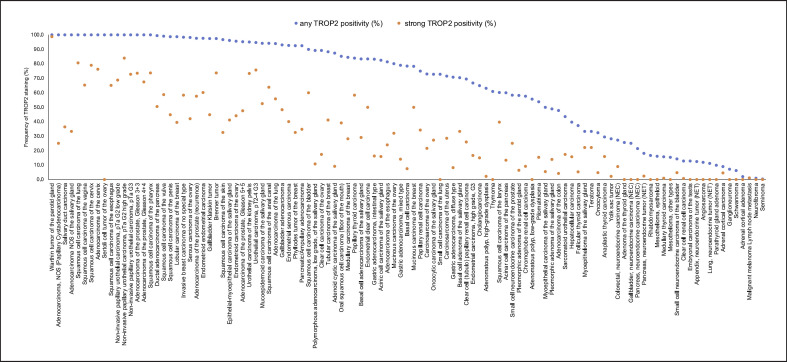
TROP2 immunostaining was detectable in 9,691 (60.5%) of the 16,024 analyzable tumors, including 2,259 (14.1%) with weak, 2,899 (18.1%) with moderate, and 4,533 (28.3%) with strong positivity. Overall, 109 (72.6%) of 150 tumor categories showed detectable TROP2 expression with 89 (59.3%) tumor categories including at least 1 case with strong positivity (Table 1). Among 86 epithelial tumor entities, 84 (97.7%) showed detectable TROP2 expression with 78 (90.7%) tumor categories including at least 1 case with strong positivity. The only exceptions were 4 cases of medullary thyroid carcinomas and one colorectal neuroendocrine tumor (NET), which did not show detectable TROP2 staining. Particularly high rates of positivity and high expression levels were seen in squamous cell carcinomas of various origins and various categories of urothelial, breast, prostate, pancreatic, and ovarian cancers. Representative images of TROP2 immunostaining in tumor tissues are shown in Figure 2. A graphical representation of a ranking order of TROP2-positive and strongly positive cancers is given in Figure 3. The relationship between TROP2 immunostaining and histopathological features is shown in Table 2. High TROP2 expression was linked to advanced stage (p = 0.0069), nodal metastasis (p < 0.0001), blood vessel (V+, p = 0.0012), and lymph vessel invasion (L1, p < 0.0001) in colorectal cancer and nodal metastasis in gastric adenocarcinoma (p = 0.0246) and papillary thyroid cancer (p = 0.0013). Low TROP2 expression was linked to advanced stage in urothelial carcinoma (p < 0.0001), high pT (p = 0.0024), high grade (p < 0.0001), and “triple negative receptor status” (p = 0.001) in breast cancer, as well as with high Fuhrmann grade (p < 0.0001) and pT stage (p = 0.0009) in papillary renal cell carcinomas. Associations between TROP2 expression and clinicopathological features were not found in clear cell renal cell carcinomas, high-grade serous ovarian carcinomas, pancreatic adenocarcinomas, and endometroid endometrial carcinomas.
Table 1.
TROP2 immunostaining in human tumors
| Tumor entity | On TMA, n | TROP2 immunostaining result |
||||||
|---|---|---|---|---|---|---|---|---|
| analyzable, n | negative, % | weak, % | moderate, % | strong, % | ||||
| Tumors of the skin | Pilomatrixoma | 35 | 26 | 46.2 | 38.5 | 0.0 | 15.4 | |
| Basal cell carcinoma | 88 | 79 | 21.5 | 54.4 | 16.5 | 7.6 | ||
| Benign nevus | 29 | 26 | 100.0 | 0.0 | 0.0 | 0.0 | ||
| Squamous cell carcinoma of the skin | 90 | 89 | 3.4 | 20.2 | 43.8 | 32.6 | ||
| Malignant melanoma | 46 | 43 | 100.0 | 0.0 | 0.0 | 0.0 | ||
| Malignant melanoma lymph node metastasis | 86 | 85 | 98.8 | 0.0 | 0.0 | 1.2 | ||
| Merkel cell carcinoma | 46 | 33 | 100.0 | 0.0 | 0.0 | 0.0 | ||
|
| ||||||||
| Tumors of the head and neck | Squamous cell carcinoma of the larynx | 110 | 88 | 39.8 | 4.5 | 15.9 | 39.8 | |
| Squamous cell carcinoma of the pharynx | 60 | 57 | 0.0 | 14.0 | 12.3 | 73.7 | ||
| Oral squamous cell carcinoma (floor of the mouth) | 130 | 115 | 14.8 | 21.7 | 24.3 | 39.1 | ||
| Pleomorphic adenoma of the parotid gland | 50 | 31 | 41.9 | 29.0 | 22.6 | 6.5 | ||
| Warthin tumor of the parotid gland | 104 | 82 | 0.0 | 0.0 | 1.2 | 98.8 | ||
| Adenocarcinoma, NOS (papillary cystadenocarcinoma) | 14 | 12 | 0.0 | 25.0 | 50.0 | 25.0 | ||
| Salivary duct carcinoma | 15 | 11 | 0.0 | 27.3 | 36.4 | 36.4 | ||
| Acinic cell carcinoma of the salivary gland | 181 | 131 | 17.6 | 38.2 | 28.2 | 16.0 | ||
| Adenocarcinoma NOS of the salivary gland | 109 | 69 | 0.0 | 20.3 | 46.4 | 33.3 | ||
| Adenoid cystic carcinoma of the salivary gland | 180 | 87 | 12.6 | 40.2 | 37.9 | 9.2 | ||
| Basal cell adenocarcinoma of the salivary gland | 25 | 24 | 16.7 | 16.7 | 37.5 | 29.2 | ||
| Basal cell adenoma of the salivary gland | 101 | 81 | 29.6 | 13.6 | 23.5 | 33.3 | ||
| Epithelial-myoepithelial carcinoma of the salivary gland | 53 | 51 | 3.9 | 7.8 | 47.1 | 41.2 | ||
| Mucoepidermoid carcinoma of the salivary gland | 343 | 242 | 5.8 | 4.5 | 37.2 | 52.5 | ||
| Myoepithelial carcinoma of the salivary gland | 21 | 18 | 50.0 | 16.7 | 27.8 | 5.6 | ||
| Myoepithelioma of the salivary gland | 11 | 9 | 66.7 | 11.1 | 0.0 | 22.2 | ||
| Oncocytic carcinoma of the salivary gland | 12 | 11 | 27.3 | 27.3 | 18.2 | 27.3 | ||
| Polymorphous adenocarcinoma, low grade, of the salivary gland | 41 | 37 | 10.8 | 10.8 | 67.6 | 10.8 | ||
| Pleomorphic adenoma of the salivary gland | 53 | 43 | 51.2 | 11.6 | 23.3 | 14.0 | ||
|
| ||||||||
| Tumors of the lung, pleura, and thymus | Adenocarcinoma of the lung | 196 | 181 | 6.1 | 4.4 | 33.7 | 55.8 | |
| Squamous cell carcinoma of the lung | 80 | 72 | 0.0 | 12.5 | 6.9 | 80.6 | ||
| Small-cell carcinoma of the lung | 16 | 11 | 27.3 | 63.6 | 9.1 | 0.0 | ||
| Mesothelioma, epithelioid | 39 | 31 | 83.9 | 12.9 | 3.2 | 0.0 | ||
| Mesothelioma, other types | 76 | 52 | 84.6 | 13.5 | 1.9 | 0.0 | ||
| Thymoma | 29 | 23 | 39.1 | 43.5 | 17.4 | 0.0 | ||
|
| ||||||||
| Tumors of the female genital tract | Squamous cell carcinoma of the vagina | 78 | 46 | 0.0 | 10.9 | 23.9 | 65.2 | |
| Squamous cell carcinoma of the vulva | 130 | 109 | 0.9 | 12.8 | 27.5 | 58.7 | ||
| Squamous cell carcinoma of the cervix | 129 | 114 | 0.0 | 2.6 | 18.4 | 78.9 | ||
| Adenocarcinoma of the cervix | 21 | 21 | 0.0 | 4.8 | 19.0 | 76.2 | ||
| Endometrioid endometrial carcinoma | 236 | 203 | 2.5 | 16.7 | 20.7 | 60.1 | ||
| Endometrial serous carcinoma | 82 | 55 | 7.3 | 18.2 | 34.5 | 40.0 | ||
| Carcinosarcoma of the uterus | 48 | 42 | 28.6 | 19.0 | 23.8 | 28.6 | ||
| Endometrial carcinoma, high grade, G3 | 13 | 12 | 33.3 | 33.3 | 16.7 | 16.7 | ||
| Endometrial clear cell carcinoma | 8 | 6 | 16.7 | 16.7 | 16.7 | 50.0 | ||
| Endometrioid carcinoma of the ovary | 110 | 93 | 4.3 | 22.6 | 29.0 | 44.1 | ||
| Serous carcinoma of the ovary | 559 | 514 | 1.8 | 29.8 | 26.5 | 42.0 | ||
| Mucinous carcinoma of the ovary | 96 | 75 | 20.0 | 16.0 | 32.0 | 32.0 | ||
| Clear cell carcinoma of the ovary | 50 | 46 | 10.9 | 47.8 | 23.9 | 17.4 | ||
| Carcinosarcoma of the ovary | 47 | 37 | 27.0 | 43.2 | 8.1 | 21.6 | ||
| Granulosa cell tumor of the ovary | 37 | 35 | 100.0 | 0.0 | 0.0 | 0.0 | ||
| Leydig cell tumor of the ovary | 4 | 4 | 100.0 | 0.0 | 0.0 | 0.0 | ||
| Sertoli cell tumor of the ovary | 1 | 1 | 0.0 | 100.0 | 0.0 | 0.0 | ||
| Sertoli Leydig cell tumor of the ovary | 3 | 3 | 100.0 | 0.0 | 0.0 | 0.0 | ||
| Steroid cell tumor of the ovary | 3 | 3 | 100.0 | 0.0 | 0.0 | 0.0 | ||
| Brenner tumor | 41 | 38 | 2.6 | 2.6 | 21.1 | 73.7 | ||
| Tumors of the breast | Invasive breast carcinoma of no special type | 1,764 | 1,594 | 1.6 | 8.8 | 31.3 | 58.3 | |
| Lobular carcinoma of the breast | 363 | 309 | 1.3 | 7.8 | 51.5 | 39.5 | ||
| Medullary carcinoma of the breast | 34 | 32 | 15.6 | 12.5 | 43.8 | 28.1 | ||
| Tubular carcinoma of the breast | 29 | 17 | 11.8 | 0.0 | 47.1 | 41.2 | ||
| Mucinous carcinoma of the breast | 65 | 46 | 21.7 | 10.9 | 17.4 | 50.0 | ||
| Phyllodes tumor of the breast | 50 | 40 | 7.5 | 0.0 | 60.0 | 32.5 | ||
|
| ||||||||
| Tumors of the digestive system | Adenomatous polyp, low-grade dysplasia | 50 | 27 | 44.4 | 51.9 | 3.7 | 0.0 | |
| Adenomatous polyp, high-grade dysplasia | 50 | 46 | 37.0 | 52.2 | 8.7 | 2.2 | ||
| Adenocarcinoma of the colon | 2,482 | 2,171 | 52.3 | 32.5 | 10.9 | 4.2 | ||
| Gastric adenocarcinoma, diffuse type | 176 | 144 | 29.2 | 20.8 | 41.7 | 8.3 | ||
| Gastric adenocarcinoma, intestinal type | 174 | 160 | 16.9 | 36.3 | 30.6 | 16.3 | ||
| Gastric adenocarcinoma, mixed type | 62 | 57 | 21.1 | 22.8 | 42.1 | 14.0 | ||
| Adenocarcinoma of the esophagus | 83 | 75 | 18.7 | 16.0 | 41.3 | 24.0 | ||
| Squamous cell carcinoma of the esophagus | 75 | 66 | 0.0 | 10.6 | 24.2 | 65.2 | ||
| Squamous cell carcinoma of the anal canal | 89 | 69 | 5.8 | 10.1 | 20.3 | 63.8 | ||
| Cholangiocarcinoma | 50 | 40 | 35.0 | 20.0 | 30.0 | 15.0 | ||
| Gallbladder adenocarcinoma | 31 | 29 | 6.9 | 10.3 | 34.5 | 48.3 | ||
| Gallbladder Klatskin tumor | 41 | 38 | 2.6 | 15.8 | 36.8 | 44.7 | ||
| Hepatocellular carcinoma | 300 | 287 | 60.3 | 10.8 | 13.2 | 15.7 | ||
| Ductal adenocarcinoma of the pancreas | 612 | 383 | 0.5 | 6.5 | 42.6 | 50.4 | ||
| Pancreatic/ampullary adenocarcinoma | 89 | 66 | 7.6 | 16.7 | 40.9 | 34.8 | ||
| Acinar cell carcinoma of the pancreas | 16 | 15 | 40.0 | 13.3 | 33.3 | 13.3 | ||
| GIST | 50 | 47 | 100.0 | 0.0 | 0.0 | 0.0 | ||
|
| ||||||||
| Tumors of the urinary system | Noninvasive papillary urothelial carcinoma, pTa G2 low grade | 177 | 125 | 0.0 | 1.6 | 29.6 | 68.8 | |
| Noninvasive papillary urothelial carcinoma, pTa G2 high grade | 141 | 106 | 0.0 | 1.9 | 14.2 | 84.0 | ||
| Noninvasive papillary urothelial carcinoma, pTa G3 | 219 | 162 | 0.0 | 4.9 | 22.2 | 72.8 | ||
| Urothelial carcinoma, pT2–4 G3 | 735 | 587 | 5.3 | 2.7 | 16.4 | 75.6 | ||
| Squamous cell carcinoma of the bladder | 22 | 20 | 10.0 | 5.0 | 25.0 | 60.0 | ||
| Small-cell NEC of the bladder | 23 | 21 | 85.7 | 4.8 | 4.8 | 4.8 | ||
| Sarcomatoid urothelial carcinoma | 25 | 23 | 56.5 | 17.4 | 8.7 | 17.4 | ||
| Urothelial carcinoma of the kidney pelvis | 62 | 60 | 5.0 | 6.7 | 15.0 | 73.3 | ||
| Clear cell renal cell carcinoma | 1,287 | 1,180 | 87.1 | 6.0 | 6.0 | 0.8 | ||
| Papillary renal cell carcinoma | 368 | 339 | 25.1 | 15.9 | 24.8 | 34.2 | ||
| Clear cell (tubulo) papillary renal cell carcinoma | 26 | 23 | 30.4 | 21.7 | 21.7 | 26.1 | ||
| Chromophobe renal cell carcinoma | 170 | 153 | 42.5 | 37.9 | 10.5 | 9.2 | ||
| Oncocytoma | 257 | 228 | 67.5 | 22.8 | 4.8 | 4.8 | ||
|
| ||||||||
| Tumors of the male genital organs | Adenocarcinoma of the prostate, Gleason 3 + 3 | 83 | 83 | 0.0 | 2.4 | 24.1 | 73.5 | |
| Adenocarcinoma of the prostate, Gleason 4 + 4 | 80 | 80 | 0.0 | 3.8 | 28.8 | 67.5 | ||
| Adenocarcinoma of the prostate, Gleason 5 + 5 | 85 | 84 | 4.8 | 10.7 | 36.9 | 47.6 | ||
| Adenocarcinoma of the prostate (recurrence) | 258 | 250 | 2.4 | 8.4 | 31.6 | 57.6 | ||
| Small-cell NEC of the prostate | 19 | 12 | 41.7 | 33.3 | 0.0 | 25.0 | ||
| Seminoma | 621 | 592 | 99.7 | 0.3 | 0.0 | 0.0 | ||
| Embryonal carcinoma of the testis | 50 | 47 | 87.2 | 10.6 | 2.1 | 0.0 | ||
| Leydig cell tumor of the testis | 30 | 30 | 100.0 | 0.0 | 0.0 | 0.0 | ||
| Sertoli cell tumor of the testis | 2 | 2 | 100.0 | 0.0 | 0.0 | 0.0 | ||
| Sex cord stromal tumor of the testis | 1 | 1 | 100.0 | 0.0 | 0.0 | 0.0 | ||
| Spermatocytic tumor of the testis | 1 | 1 | 100.0 | 0.0 | 0.0 | 0.0 | ||
| Yolk sac tumor | 50 | 46 | 71.7 | 26.1 | 0.0 | 2.2 | ||
| Teratoma | 50 | 36 | 66.7 | 2.8 | 8.3 | 22.2 | ||
| Squamous cell carcinoma of the penis | 80 | 78 | 1.3 | 14.1 | 39.7 | 44.9 | ||
| Tumors of endocrine organs | Adenoma of the thyroid gland | 114 | 113 | 74.3 | 20.4 | 5.3 | 0.0 | |
| Papillary thyroid carcinoma | 392 | 374 | 16.0 | 13.9 | 11.8 | 58.3 | ||
| Follicular thyroid carcinoma | 154 | 150 | 62.7 | 19.3 | 12.7 | 5.3 | ||
| Medullary thyroid carcinoma | 111 | 108 | 84.3 | 13.9 | 0.9 | 0.9 | ||
| Parathyroid gland adenoma | 43 | 40 | 90.0 | 7.5 | 2.5 | 0.0 | ||
| Anaplastic thyroid carcinoma | 45 | 44 | 70.5 | 9.1 | 4.5 | 15.9 | ||
| Adrenal cortical adenoma | 50 | 47 | 97.9 | 0.0 | 2.1 | 0.0 | ||
| Adrenal cortical carcinoma | 26 | 22 | 90.9 | 4.5 | 0.0 | 4.5 | ||
| Phaeochromocytoma | 50 | 47 | 100.0 | 0.0 | 0.0 | 0.0 | ||
| Appendix, NET | 22 | 16 | 87.5 | 12.5 | 0.0 | 0.0 | ||
| Colorectal, NET | 12 | 11 | 100.0 | 0.0 | 0.0 | 0.0 | ||
| Ileum, NET | 49 | 49 | 100.0 | 0.0 | 0.0 | 0.0 | ||
| Lung, NET | 19 | 18 | 88.9 | 5.6 | 5.6 | 0.0 | ||
| Pancreas, NET | 97 | 83 | 81.9 | 12.0 | 3.6 | 2.4 | ||
|
| ||||||||
| Colorectal, NEC | 12 | 11 | 72.7 | 9.1 | 9.1 | 9.1 | ||
| Gallbladder, NEC | 4 | 4 | 75.0 | 25.0 | 0.0 | 0.0 | ||
| Pancreas, NEC | 14 | 14 | 78.6 | 21.4 | 0.0 | 0.0 | ||
| Tumors of hematopoietic and lymphoid tissuesw | Hodgkin lymphoma | 103 | 78 | 100.0 | 0.0 | 0.0 | 0.0 | |
| B-SLL/B-CLL | 50 | 47 | 100.0 | 0.0 | 0.0 | 0.0 | ||
| DLBCL | 114 | 108 | 100.0 | 0.0 | 0.0 | 0.0 | ||
| Follicular lymphoma | 88 | 87 | 100.0 | 0.0 | 0.0 | 0.0 | ||
| T-cell non-Hodgkin lymphoma | 24 | 24 | 100.0 | 0.0 | 0.0 | 0.0 | ||
| Mantle cell lymphoma | 18 | 18 | 100.0 | 0.0 | 0.0 | 0.0 | ||
| Marginal zone lymphoma | 16 | 15 | 100.0 | 0.0 | 0.0 | 0.0 | ||
| DLBCL in the testis | 16 | 16 | 100.0 | 0.0 | 0.0 | 0.0 | ||
| Burkitt lymphoma | 5 | 3 | 100.0 | 0.0 | 0.0 | 0.0 | ||
|
| ||||||||
| Tumors of soft tissue and bone | Tenosynovial giant cell tumor | 45 | 29 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Granular cell tumor | 53 | 32 | 100.0 | 0.0 | 0.0 | 0.0 | ||
| Leiomyoma | 50 | 49 | 100.0 | 0.0 | 0.0 | 0.0 | ||
| Leiomyosarcoma | 87 | 76 | 100.0 | 0.0 | 0.0 | 0.0 | ||
| Liposarcoma | 132 | 112 | 100.0 | 0.0 | 0.0 | 0.0 | ||
| MPNST | 13 | 13 | 100.0 | 0.0 | 0.0 | 0.0 | ||
| Myofibrosarcoma | 26 | 26 | 100.0 | 0.0 | 0.0 | 0.0 | ||
| Angiosarcoma | 73 | 59 | 88.1 | 11.9 | 0.0 | 0.0 | ||
| Angiomyolipoma | 91 | 88 | 100.0 | 0.0 | 0.0 | 0.0 | ||
| Dermatofibrosarcoma protuberans | 21 | 17 | 100.0 | 0.0 | 0.0 | 0.0 | ||
| Ganglioneuroma | 14 | 14 | 92.9 | 7.1 | 0.0 | 0.0 | ||
| Kaposi sarcoma | 8 | 5 | 100.0 | 0.0 | 0.0 | 0.0 | ||
| Neurofibroma | 117 | 104 | 99.0 | 1.0 | 0.0 | 0.0 | ||
| Sarcoma, NOS | 74 | 72 | 100.0 | 0.0 | 0.0 | 0.0 | ||
| Paraganglioma | 41 | 41 | 100.0 | 0.0 | 0.0 | 0.0 | ||
| Ewing sarcoma | 23 | 18 | 100.0 | 0.0 | 0.0 | 0.0 | ||
| Rhabdomyosarcoma | 6 | 6 | 83.3 | 16.7 | 0.0 | 0.0 | ||
| Schwannoma | 121 | 113 | 93.8 | 6.2 | 0.0 | 0.0 | ||
| Synovial sarcoma | 12 | 11 | 100.0 | 0.0 | 0.0 | 0.0 | ||
| Osteosarcoma | 43 | 34 | 100.0 | 0.0 | 0.0 | 0.0 | ||
| Chondrosarcoma | 38 | 19 | 100.0 | 0.0 | 0.0 | 0.0 | ||
| Rhabdoid tumor | 5 | 5 | 100.0 | 0.0 | 0.0 | 0.0 | ||
NOS, not otherwise specified; MPNST, malignant peripheral nerve sheath tumor; DLBCL, diffuse large B-cell lymphoma; B-SLL/B-CLL, small lymphocytic lymphoma, B-cell type; NEC, neuroendocrine carcinoma; GIST, gastrointestinal stromal tumor.
Fig. 2.
TROP2 immunostaining in cancer. The panels show a strong, membranous, and cytoplasmatic TROP2 immunostaining in a squamous cell carcinoma of the oral cavity (a), a recurrent adenocarcinoma (Gleason 5 + 5 = 10) of the prostate (b), a breast cancer of no special type (c), a gastric adenocarcinoma (d), a papillary carcinoma of the thyroid (e), and an adenocarcinoma of the lung (f). TROP2 staining is absent in an epithelioid pleural mesothelioma (g) and a colorectal adenocarcinoma (h).
Fig. 3.
Ranking order of TROP2 immunostaining in cancers. Both the frequency of positive cases (blue dots) and the frequency of strongly positive cases (orange dots) are shown.
Table 2.
TROP2 immunostaining and tumor phenotype in colon, papillary thyroid, breast, urinary bladder, stomach, and papillary kidney carcinomas
| n | TROP2 immunostaining result |
p value | ||||
|---|---|---|---|---|---|---|
| negative, % | weak, % | moderate, % | strong, % | |||
| Colon adenocarcinoma | ||||||
| Primary tumor | ||||||
| pT1 | 79 | 54.4 | 35.4 | 8.9 | 1.3 | 0.0069 |
| pT2 | 406 | 53.2 | 34.5 | 7.4 | 4.9 | |
| pT3 | 1,157 | 53.5 | 32.2 | 11.2 | 3.1 | |
| pT4 | 419 | 49.6 | 30.1 | 13.4 | 6.9 | |
| Regional lymph nodes | ||||||
| pN0 | 1,088 | 57.9 | 30.6 | 8.1 | 3.4 | <0.0001 |
| pN+ | 963 | 46.6 | 34.2 | 14.1 | 5.1 | |
| Venous invasion | ||||||
| V0 | 1,479 | 54.9 | 31.3 | 9.6 | 4.2 | 0.0012 |
| V1 | 543 | 45.9 | 35.5 | 14.2 | 4.4 | |
| Lymphatic invasion | ||||||
| L0 | 661 | 58.9 | 31.2 | 7 | 3 | <0.0001 |
| L1 | 1,368 | 49.3 | 33 | 12.9 | 4.8 | |
| Tumor localization | ||||||
| Left colon | 1,122 | 52 | 35 | 8.9 | 4.1 | 0.0273 |
| Right colon | 425 | 53.2 | 29.2 | 13.4 | 4.2 | |
| MMR status | ||||||
| Defective | 86 | 51.2 | 34.9 | 10.5 | 3.5 | 0.9848 |
| Proficient | 1,071 | 51.1 | 35.5 | 9.4 | 4 | |
| RAS mutation status | ||||||
| Mutated | 325 | 48.3 | 38.5 | 9.8 | 3.4 | 0.2722 |
| Wild type | 414 | 54.8 | 32.6 | 8.5 | 4.1 | |
| BRAF mutation status | ||||||
| Mutated | 14 | 42.9 | 14.3 | 28.6 | 14.3 | 0.1262 |
| Wild type | 90 | 56.7 | 28.9 | 10 | 4.4 | |
| Papillary thyroid carcinomas | ||||||
| Primary tumor | ||||||
| pT1 | 151 | 11.9 | 17.9 | 9.3 | 60.9 | 0.0487 |
| pT2 | 76 | 26.3 | 14.5 | 11.8 | 47.4 | |
| pT3–4 | 96 | 12.5 | 11.5 | 7.3 | 68.8 | |
| Regional lymph nodes | ||||||
| pN0 | 89 | 20.2 | 13.5 | 7.9 | 58.4 | 0.0013 |
| pN+ | 122 | 4.1 | 10.7 | 7.4 | 77.9 | |
| Breast carcinoma of no special type | ||||||
| Primary tumor | ||||||
| pT1 | 899 | 0.9 | 6.8 | 30.1 | 62.2 | 0.0024 |
| pT2 | 796 | 2 | 8.3 | 33.5 | 56.2 | |
| pT3–4 | 182 | 4.9 | 8.8 | 35.2 | 51.1 | |
| Grade | ||||||
| G1 | 215 | 0.5 | 2.8 | 22.3 | 74.4 | <0.0001 |
| G2 | 1,050 | 1.8 | 5.6 | 33.2 | 59.3 | |
| G3 | 659 | 2.3 | 12.3 | 33.2 | 52.2 | |
| Regional lymph nodes | ||||||
| pN0 | 872 | 1.9 | 7.1 | 32.3 | 58.6 | 0.286 |
| pN1 | 406 | 1.5 | 8.6 | 31.3 | 58.6 | |
| pN2 | 148 | 1.4 | 6.1 | 35.8 | 56.8 | |
| pN3 | 100 | 4 | 6 | 44 | 46 | |
| HER2 status | ||||||
| Negative | 995 | 1.9 | 9.9 | 30.3 | 57.9 | 0.6044 |
| Positive | 125 | 0.8 | 12 | 32.8 | 54.4 | |
| ER status | ||||||
| Negative | 233 | 1.7 | 19.7 | 24.9 | 53.6 | <0.0001 |
| Positive | 822 | 1.9 | 8 | 31.4 | 58.6 | |
| PR status | ||||||
| Negative | 457 | 2.2 | 13.1 | 29.5 | 55.1 | 0.0305 |
| Positive | 654 | 1.7 | 7.8 | 31.5 | 59 | |
| Triple negative | ||||||
| No | 858 | 2 | 9 | 31.2 | 57.8 | 0.001 |
| Yes | 158 | 1.3 | 20.3 | 24.1 | 54.4 | |
| Urinary bladder carcinoma | ||||||
| Primary tumor | ||||||
| pTa G2 low | 125 | 0 | 1.6 | 29.6 | 68.8 | <0.0001 |
| pTa G2 high | 106 | 0 | 1.9 | 14.2 | 84 | |
| pTa G3 | 133 | 0.8 | 6 | 26.3 | 66.9 | |
| pT2 | 122 | 3.3 | 0.8 | 13.9 | 82 | |
| pT3 | 203 | 3 | 3.4 | 17.2 | 76.4 | |
| pT4 | 97 | 8.2 | 2.1 | 16.5 | 73.2 | |
| Regional lymph nodes | ||||||
| pN0 | 242 | 3.7 | 2.1 | 16.1 | 78.1 | 0.9068 |
| pN+ | 170 | 3.2 | 3.2 | 16 | 77.6 | |
| Gastric carcinoma | ||||||
| Laurén type | ||||||
| Diffuse | 66 | 30.3 | 24.2 | 43.9 | 1.5 | 0.0208 |
| Intestinal | 81 | 22.2 | 38.3 | 29.6 | 9.9 | |
| Mixed | 57 | 21.1 | 22.8 | 42.1 | 14 | |
| Tumor stage | ||||||
| pT1–2 | 48 | 35.4 | 27.1 | 29.2 | 8.3 | 0.1352 |
| pT3 | 114 | 22.8 | 21.9 | 42.1 | 13.2 | |
| pT4 | 111 | 17.1 | 27.9 | 46.8 | 8.1 | |
| Regional lymph nodes | ||||||
| pN0 | 69 | 31.9 | 30.4 | 30.4 | 7.2 | 0.0246 |
| pN1 | 58 | 27.6 | 19 | 39.7 | 13.8 | |
| pN2 | 55 | 16.4 | 18.2 | 47.3 | 18.2 | |
| pN3 | 90 | 16.7 | 30 | 47.8 | 5.6 | |
| Mismatch repair status | ||||||
| MMR defective | 32 | 46.9 | 18.8 | 18.8 | 15.6 | 0.0002 |
| MMR proficient | 233 | 14.6 | 24.9 | 48.9 | 11.6 | |
| Papillary renal cell carcinomas | ||||||
| ISUP stage | ||||||
| 1 | 41 | 17.1 | 22 | 19.5 | 41.5 | 0.0005 |
| 2 | 134 | 14.9 | 17.2 | 25.4 | 42.5 | |
| 3 | 81 | 39.5 | 14.8 | 28.4 | 17.3 | |
| 4 | 7 | 57.1 | 14.3 | 14.3 | 14.3 | |
| Fuhrmann grade | ||||||
| 1 | 4 | 0 | 50 | 0 | 50 | <0.0001 |
| 2 | 183 | 14.2 | 17.5 | 25.1 | 43.2 | |
| 3 | 83 | 41 | 18.1 | 24.1 | 16.9 | |
| 4 | 11 | 45.5 | 9.1 | 36.4 | 9.1 | |
| Thoenes grade | ||||||
| 1 | 58 | 13.8 | 17.2 | 22.4 | 46.6 | 0.1706 |
| 2 | 157 | 26.1 | 17.8 | 24.2 | 31.8 | |
| 3 | 18 | 33.3 | 16.7 | 33.3 | 16.7 | |
| n | TROP2 immunostaining result | p value | ||||
| negative, % | weak, % | moderate, % | strong, % | |||
| UICC stage | ||||||
| 1 | 102 | 23.5 | 17.6 | 22.5 | 36.3 | 0.0097 |
| 2 | 15 | 13.3 | 13.3 | 46.7 | 26.7 | |
| 3 | 5 | 80 | 0 | 0 | 20 | |
| 4 | 11 | 36.4 | 0 | 54.5 | 9.1 | |
| Primary tumor | ||||||
| 1 | 208 | 21.2 | 17.8 | 24.5 | 36.5 | 0.0009 |
| 2 | 48 | 10.4 | 18.8 | 33.3 | 37.5 | |
| 3–4 | 33 | 54.5 | 9.1 | 21.2 | 15.2 | |
| Regional lymph nodes | ||||||
| 0 | 25 | 36 | 4 | 28 | 32 | 0.4776 |
| ≥1 | 15 | 33.3 | 6.7 | 46.7 | 13.3 | |
| Distant metastasis | ||||||
| 0 | 27 | 25.9 | 11.1 | 25.9 | 37 | 0.2267 |
| ≥1 | 12 | 41.7 | 8.3 | 41.7 | 8.3 | |
Discussion
Considering the large scale of our study, emphasis was placed on the appropriate validation of our assay. The International Working Group for Antibody Validation (IWGAV) has proposed that antibody validation for IHC on formalin-fixed tissues should include either a comparison of the findings obtained by two different independent antibodies or a comparison with expression data obtained by another independent method [37]. Seventy-six different normal tissue categories were utilized for assay validation in this study. This broad range of tissues is likely to contain most proteins that are normally expressed at relevant levels in cells of adult humans and should therefore enable the detection of most undesired cross-reactivities of tested antibodies. RNA expression data derived from three independent RNA screening studies, including the Human Protein Atlas (HPA) RNA-seq tissue dataset [38], the FANTOM5 project [39, 40], and the Genotype-Tissue Expression (GTEx) project, had shown particularly high TROP2 expression levels in all tissues covered by squamous epithelium and salivary glands and − at somewhat lower levels − also in breast, prostate, salivary glands, and the urinary bladder [41]. These observations were in concordance with our IHC data. Other cell types that also showed distinct TROP2 immunostaining such as thymic epithelial cells, respiratory epithelium, bronchial glands, amnion and chorion cells of the placenta, superficial epithelial cell layers of the stomach, few scattered epithelial cells in the intestine, gallbladder epithelium, intrahepatic bile ducts as well as of a fraction of cells in the fallopian tube, endometrium, thyroid, and adenohypophysis were all confirmed by the independent second antibody approach (R&D AF650; online suppl. Fig. 1). In all these organs, the TROP2-positive cell populations constitute rather small subsets of the total amount of cells and may thus not have resulted in conspicuous findings in RNA analyses. The widespread expression of TROP2 in a broad range of epithelial cell types is comparable to the expression pattern of its family member TACSTD1 (EpCAM). Antibodies against EpCAM are widely used in routine pathology where they serve as a marker for epithelial cell origin [42]. The main difference between TROP2 and EpCAM is the limitation of TROP2 expression to only few specific cell types in the GIT, whereas EpCAM is abundantly expressed in all epithelial cells of the GIT [43]. The low expression of TROP2 in the GIT may reflect one of the causes for the less severe side effects of anti-TROP2 drugs as compared to various earlier anti-EpCAM drug candidates, many of which earlier failed for systemic therapy [44].
The reported response rates to SG ranged between 19% [13] and 33.3% in clinical trials [15]. Since particularly high levels of TROP2 expression have so far not been required for including patients into clinical trials, it appears possible that absent or very low levels of TROP2 expression might have contributed to some of the therapy failures. Studies performing retrospective analyses of TROP2 expression in cancers treated by SG have indeed described better response rates in strongly TROP2-positive cancers as compared to low expressors [12, 30, 31]. It appears therefore likely that the ranking order of tumor types according to their frequency and intensity of TROP2 immunostaining which was generated in this study might delineate tumor entities that might be particularly suitable for SG therapy. Although our data show that TROP2 expression − often at high level − can be found in virtually all epithelial tumor entities, they do identify several important cancer entities that are particularly prone to high-level TROP2 expression such as squamous cell carcinomas of various origins, urothelial, breast, and ovarian cancers, as well as primary and recurrent adenocarcinomas of the prostate. It is of note that triple negative breast cancer, one of the few cancer types for which SG is currently FDA approved, did not range among the top-ranked TROP2 expressing cancers and only included 54.4% strongly positive cases. Triple negative breast cancer with progression on at least two prior therapies was probably not selected for early SG trials based on their particularly high rate of TROP2 expression but rather because of the lack of further therapeutic options. As compared to triple negative breast cancer, frequency and levels of TROP2 positivity were higher in advanced prostate cancers with recurrence under systemic therapy suggesting that these tumors might also be suitable candidates for SG therapy. Clinical trials applying SG in castration-resistant prostate cancer are ongoing [45]. Other studies investigate the use of SG in urothelial carcinoma [14], nonsmall-cell lung cancer [13], and small-cell lung cancer [12].
The comprehensive analysis of relevant tumor entities for expression of a specific protein also enabled an assessment of the potential diagnostic utility of an antibody in surgical pathology. Based on the marked difference in TROP2 expression between epithelioid mesothelioma (16.1%) and adenocarcinomas of the lung (93.9%), TROP2 IHC could be of use for the difficult distinction of these tumor entities. This differential diagnosis regularly requires the use of diagnostic antibody panels which currently often include BAP-1, WT-1, D2-40, calretinin, EpCAM, TTF-1, and claudin-4 [46]. A low or absent TROP2 expression in an “intestinal-type” adenocarcinoma could favor the diagnosis of colorectal adenocarcinoma (negative or weak TROP2 expression in 84.8%) as morphologically similar tumors such as ductal adenocarcinomas of the pancreas (7% negative/weak), adenocarcinomas of the gall bladder (17.2% negative/weak), and even gastric carcinomas (45–50% negative/weak) typically show low TROP2 expression levels less commonly. Moreover, a high TROP2 expression may favor a diagnosis of papillary over follicular carcinoma of the thyroid. These potential diagnostic applications of TROP2 IHC need to be evaluated in further studies.
The large number of tumors analyzed within several of our tumor categories also enabled an analysis of the potential clinical significance of variable TROP2 expression levels for these tumor entities. The significant association of high TROP2 expression levels with stage progression, nodal metastasis, L1, and V1 status in colorectal carcinomas and with nodal metastasis in papillary thyroid cancers demonstrates that upregulation of TROP2 in a cancer derived from TROP2-negative precursor cells can play a role in cancer progression. Fang et al. [34] and Zhao et al. [47] have also reported an association between high TROP2 expression and unfavorable tumor features in studies on 82–620 colon cancers, and Abdou et al. [48] found a link between TROP2 positivity and lymph node metastasis in 56 papillary thyroid cancers. The significant associations between low TROP2 expression and advanced pT stage and high grade in breast and papillary kidney cancer demonstrate, however, that reduced TROP2 expression in a cancer derived from TROP2-positive precursor cells can also be linked to unfavorable tumor features. Only one study has analyzed the prognostic value of TROP2 in breast cancer as to yet. Ambrogi et al. [33] reported an association between membranous TROP2 expression and poor patient outcome, while merely intracellular TROP2 had a favorable prognostic impact in a study on 702 breast cancers.
Conclusion
Our data show that TROP2 is abundantly expressed in many normal epithelial cell types and in most epithelial neoplasms. Aberrant TROP2 expression including both upregulation and downregulation can be associated with cancer progression in a tumor-type dependent manner. Since anti-TROP2 cancer drugs have demonstrated efficiency and induce tolerable side effects, it can be assumed that these drugs will be applicable to a broad range of tumor entities in the future.
Statement of Ethics
The usage of archived diagnostic leftover tissues for manufacturing of TMAs and their analysis for research purposes as well as patient data analysis has been approved by local laws (HmbKHG, §12.1) and by the Local Ethics Committee (Ethics Commission Hamburg, WF-049/09). All work has been carried out in compliance with the Helsinki Declaration.
Conflict of Interest Statement
The TROP2 antibody clone MSVA-733R was provided from MS Validated Antibodies GmbH (owned by a family member of GS).
Funding Sources
No funding was received.
Author Contributions
D.D., N.T., C.V., W.W., R.S., G.S., A.M., and A.M.L. contributed to conception, design, data collection, data analysis, and manuscript writing. A.H., S.M., M.L., A.M.L., E.B., T.S.C., W.W., C.B., P.L., T.K., and S.S. participated in pathology data analysis and data interpretation. C.F., R.U., N.G., F.J., and S.M.: IHC analysis. A.H.M. and T.K.: conception and design, and collection of samples. C.H.-M. and R.S. performed statistical analysis. D.D., A.M.L., W.W., R.S., and G.S.: study supervision.
Data Availability Statement
Raw data are available upon reasonable request. All data relevant to the study are included in the article. All authors agree to be accountable for the content of the work.
Supplementary Material
Supplementary data
Supplementary data
Supplementary data
Acknowledgments
We are grateful to Melanie Witt, Inge Brandt, Maren Eisenberg, and Sünje Seekamp for excellent technical assistance.
References
- 1.Lin JC, Wu YY, Wu JY, Lin TC, Wu CT, Chang YL, et al. TROP2 is epigenetically inactivated and modulates IGF-1R signalling in lung adenocarcinoma. EMBO Mol Med. 2012;4((6)):472–85. doi: 10.1002/emmm.201200222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fornaro M, Dell'Arciprete R, Stella M, Bucci C, Nutini M, Capri MG, et al. Cloning of the gene encoding Trop-2, a cell-surface glycoprotein expressed by human carcinomas. Int J Cancer. 1995;62((5)):610–8. doi: 10.1002/ijc.2910620520. [DOI] [PubMed] [Google Scholar]
- 3.Trerotola M, Cantanelli P, Guerra E, Tripaldi R, Aloisi AL, Bonasera V, et al. Upregulation of Trop-2 quantitatively stimulates human cancer growth. Oncogene. 2013;32((2)):222–33. doi: 10.1038/onc.2012.36. [DOI] [PubMed] [Google Scholar]
- 4.Cardillo TM, Govindan SV, Sharkey RM, Trisal P, Goldenberg DM. Humanized anti-Trop-2 IgG-SN-38 conjugate for effective treatment of diverse epithelial cancers: preclinical studies in human cancer xenograft models and monkeys. Clin Cancer Res. 2011;17((10)):3157–69. doi: 10.1158/1078-0432.CCR-10-2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cubas R, Li M, Chen C, Yao Q. Trop2: a possible therapeutic target for late stage epithelial carcinomas. Biochim Biophys Acta. 2009;1796((2)):309–14. doi: 10.1016/j.bbcan.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Zaman S, Jadid H, Denson AC, Gray JE. Targeting Trop-2 in solid tumors: future prospects. Onco Targets Ther. 2019;12:1781–90. doi: 10.2147/OTT.S162447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guerra E, Trerotola M, Aloisi AL, Tripaldi R, Vacca G, La Sorda R, et al. The Trop-2 signalling network in cancer growth. Oncogene. 2013;32((12)):1594–600. doi: 10.1038/onc.2012.151. [DOI] [PubMed] [Google Scholar]
- 8.Liu T, Liu Y, Bao X, Tian J, Liu Y, Yang X. Overexpression of TROP2 predicts poor prognosis of patients with cervical cancer and promotes the proliferation and invasion of cervical cancer cells by regulating ERK signaling pathway. PLoS One. 2013;8((9)):e75864. doi: 10.1371/journal.pone.0075864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seligson JM, Patron AM, Berger MJ, Harvey RD, Seligson ND. Sacituzumab govitecan-hziy: an antibody-drug conjugate for the treatment of refractory, metastatic, triple-negative breast cancer. Ann Pharmacother. 2021;55((7)):921–31. doi: 10.1177/1060028020966548. [DOI] [PubMed] [Google Scholar]
- 10.Bardia A, Hurvitz SA, Rugo HS, Brufsky A, Cortes J, Loibl S, et al. A plain language summary of the ASCENT study: sacituzumab govitecan for metastatic triple-negative breast cancer. Future Oncol. 2021 Oct 1;17((30)):3911–24. doi: 10.2217/fon-2021-0868. [DOI] [PubMed] [Google Scholar]
- 11.Kalinsky K, Diamond JR, Vahdat LT, Tolaney SM, Juric D, O'Shaughnessy J, et al. Sacituzumab govitecan in previously treated hormone receptor-positive/HER2-negative metastatic breast cancer: final results from a phase I/II, single-arm, basket trial. Ann Oncol. 2020;31((12)):1709–18. doi: 10.1016/j.annonc.2020.09.004. [DOI] [PubMed] [Google Scholar]
- 12.Gray JE, Heist RS, Starodub AN, Camidge DR, Kio EA, Masters GA, et al. Therapy of small cell lung cancer (SCLC) with a topoisomerase-I-inhibiting antibody-drug conjugate (ADC) targeting Trop-2, sacituzumab govitecan. Clin Cancer Res. 2017;23((19)):5711–9. doi: 10.1158/1078-0432.CCR-17-0933. [DOI] [PubMed] [Google Scholar]
- 13.Heist RS, Guarino MJ, Masters G, Purcell WT, Starodub AN, Horn L, et al. Therapy of advanced non-small-cell lung cancer with an SN-38-Anti-Trop-2 drug conjugate, sacituzumab govitecan. J Clin Oncol. 2017;35((24)):2790–7. doi: 10.1200/JCO.2016.72.1894. [DOI] [PubMed] [Google Scholar]
- 14.Faltas B, Goldenberg DM, Ocean AJ, Govindan SV, Wilhelm F, Sharkey RM, et al. Sacituzumab govitecan, a novel antibody − drug conjugate, in patients with metastatic platinum-resistant urothelial carcinoma. Clin Genitourin Cancer. 2016;14((1)):e75–9. doi: 10.1016/j.clgc.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Bardia A, Mayer IA, Vahdat LT, Tolaney SM, Isakoff SJ, Diamond JR, et al. Sacituzumab govitecan-hziy in refractory metastatic triple-negative breast cancer. N Engl J Med. 2019;380((8)):741–51. doi: 10.1056/NEJMoa1814213. [DOI] [PubMed] [Google Scholar]
- 16.Tagawa ST, Balar AV, Petrylak DP, Kalebasty AR, Loriot Y, Flechon A, et al. TROPHY-U-01: a phase II open-label study of sacituzumab govitecan in patients with metastatic urothelial carcinoma progressing after platinum-based chemotherapy and checkpoint inhibitors. J Clin Oncol. 2021;39((22)):2474–85. doi: 10.1200/JCO.20.03489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Avellini C, Licini C, Lazzarini R, Gesuita R, Guerra E, Tossetta G, et al. The trophoblast cell surface antigen 2 and miR-125b axis in urothelial bladder cancer. Oncotarget. 2017;8((35)):58642–53. doi: 10.18632/oncotarget.17407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolber P, Nachtsheim L, Hoffmann F, Klussmann JP, Meyer M, von Eggeling F, et al. Trophoblast cell surface antigen 2 (Trop-2) protein is highly expressed in salivary gland carcinomas and represents a potential therapeutic target. Head Neck Pathol. 2021;15((4)):1147–55. doi: 10.1007/s12105-021-01325-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu N, Zhang Z, Zhu J, Xu L, Li Y, Duan L, et al. Overexpression of trophoblast cell surface antigen 2 as an independent marker for a poor prognosis and as a potential therapeutic target in epithelial ovarian carcinoma. Int J Exp Pathol. 2016;97((2)):150–8. doi: 10.1111/iep.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bignotti E, Todeschini P, Calza S, Falchetti M, Ravanini M, Tassi RA, et al. Trop-2 overexpression as an independent marker for poor overall survival in ovarian carcinoma patients. Eur J Cancer. 2010;46((5)):944–53. doi: 10.1016/j.ejca.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 21.Perrone E, Manara P, Lopez S, Bellone S, Bonazzoli E, Manzano A, et al. Sacituzumab govitecan, an antibody-drug conjugate targeting trophoblast cell-surface antigen 2, shows cytotoxic activity against poorly differentiated endometrial adenocarcinomas in vitro and in vivo. Mol Oncol. 2020;14((3)):645–56. doi: 10.1002/1878-0261.12627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han C, Perrone E, Zeybek B, Bellone S, Tymon-Rosario J, Altwerger G, et al. In vitro and in vivo activity of sacituzumab govitecan, an antibody-drug conjugate targeting trophoblast cell-surface antigen 2 (Trop-2) in uterine serous carcinoma. Gynecol Oncol. 2020;156((2)):430–8. doi: 10.1016/j.ygyno.2019.11.018. [DOI] [PubMed] [Google Scholar]
- 23.Kushiyama S, Yashiro M, Yamamoto Y, Sera T, Sugimoto A, Nishimura S, et al. Clinicopathologic significance of TROP2 and phospho-TROP2 in gastric cancer. Mol Clin Oncol. 2021;14((5)):105. doi: 10.3892/mco.2021.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao W, Ding G, Wen J, Tang Q, Yong H, Zhu H, et al. Correlation between Trop2 and amphiregulin coexpression and overall survival in gastric cancer. Cancer Med. 2017;6((5)):994–1001. doi: 10.1002/cam4.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao W, Zhu H, Zhang S, Yong H, Wang W, Zhou Y, et al. Trop2 is overexpressed in gastric cancer and predicts poor prognosis. Oncotarget. 2016;7((5)):6136–45. doi: 10.18632/oncotarget.6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang G, Tang Q, Jia L, Xia S, Li J, Chen Y, et al. High expression of TROP2 is correlated with poor prognosis of oral squamous cell carcinoma. Pathol Res Pract. 2018;214((10)):1606–12. doi: 10.1016/j.prp.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 27.Zhang B, Gao S, Li R, Li Y, Cao R, Cheng J, et al. Tissue mechanics and expression of TROP2 in oral squamous cell carcinoma with varying differentiation. BMC Cancer. 2020;20((1)):815. doi: 10.1186/s12885-020-07257-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mito R, Matsubara E, Komohara Y, Shinchi Y, Sato K, Yoshii D, et al. Clinical impact of TROP2 in non-small lung cancers and its correlation with abnormal p53 nuclear accumulation. Pathol Int. 2020;70((5)):287–94. doi: 10.1111/pin.12911. [DOI] [PubMed] [Google Scholar]
- 29.Zeybek B, Manzano A, Bianchi A, Bonazzoli E, Bellone S, Buza N, et al. Cervical carcinomas that overexpress human trophoblast cell-surface marker (Trop-2) are highly sensitive to the antibody-drug conjugate sacituzumab govitecan. Sci Rep. 2020;10((1)):973. doi: 10.1038/s41598-020-58009-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cardillo TM, Rossi DL, Zalath MB, Liu D, Arrojo R, Sharkey RM, et al. Predictive biomarkers for sacituzumab govitecan efficacy in Trop-2-expressing triple-negative breast cancer. Oncotarget. 2020;11((43)):3849–62. doi: 10.18632/oncotarget.27766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bardia A, Mayer IA, Diamond JR, Moroose RL, Isakoff SJ, Starodub AN, et al. Efficacy and safety of Anti-Trop-2 antibody drug conjugate sacituzumab govitecan (IMMU-132) in heavily pretreated patients with metastatic triple-negative breast cancer. J Clin Oncol. 2017;35((19)):2141–8. doi: 10.1200/JCO.2016.70.8297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barrett T, Wilhite SE, Ledoux P, Evangelista C, Kim IF, Tomashevsky M, et al. NCBI GEO: archive for functional genomics data sets − update. Nucleic Acids Res. 2013;41((Database issue)):D991–5. doi: 10.1093/nar/gks1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ambrogi F, Fornili M, Boracchi P, Trerotola M, Relli V, Simeone P, et al. Trop-2 is a determinant of breast cancer survival. PLoS One. 2014;9((5)):e96993. doi: 10.1371/journal.pone.0096993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fang YJ, Lu ZH, Wang GQ, Pan ZZ, Zhou ZW, Yun JP, et al. Elevated expressions of MMP7, TROP2, and survivin are associated with survival, disease recurrence, and liver metastasis of colon cancer. Int J Colorectal Dis. 2009;24((8)):875–84. doi: 10.1007/s00384-009-0725-z. [DOI] [PubMed] [Google Scholar]
- 35.Dancau AM, Simon R, Mirlacher M, Sauter G. Tissue microarrays. Methods Mol Biol. 2016;1381:53–65. doi: 10.1007/978-1-4939-3204-7_3. [DOI] [PubMed] [Google Scholar]
- 36.Kononen J, Bubendorf L, Kallioniemi A, Barlund M, Schraml P, Leighton S, et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4((7)):844–7. doi: 10.1038/nm0798-844. [DOI] [PubMed] [Google Scholar]
- 37.Uhlen M, Bandrowski A, Carr S, Edwards A, Ellenberg J, Lundberg E, et al. A proposal for validation of antibodies. Nat Methods. 2016;13((10)):823–7. doi: 10.1038/nmeth.3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thul PJ, Akesson L, Wiking M, Mahdessian D, Geladaki A, Ait Blal H, et al. A subcellular map of the human proteome. Science. 2017;356((6340)):eaal3321. doi: 10.1126/science.aal3321. [DOI] [PubMed] [Google Scholar]
- 39.Lizio M, Abugessaisa I, Noguchi S, Kondo A, Hasegawa A, Hon CC, et al. Update of the FANTOM web resource: expansion to provide additional transcriptome atlases. Nucleic Acids Res. 2019;47((D1)):D752–D8. doi: 10.1093/nar/gky1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lizio M, Harshbarger J, Shimoji H, Severin J, Kasukawa T, Sahin S, et al. Gateways to the FANTOM5 promoter level mammalian expression atlas. Genome Biol. 2015;16:22. doi: 10.1186/s13059-014-0560-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.GTEx Consortium The genotype-tissue expression (GTEx) project. Nat Genet. 2013;45((6)):580–5. doi: 10.1038/ng.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trzpis M, McLaughlin PM, de Leij LM, Harmsen MC. Epithelial cell adhesion molecule: more than a carcinoma marker and adhesion molecule. Am J Pathol. 2007;171((2)):386–95. doi: 10.2353/ajpath.2007.070152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schnell U, Cirulli V, Giepmans BN. EpCAM: structure and function in health and disease. Biochim Biophys Acta. 2013;1828((8)):1989–2001. doi: 10.1016/j.bbamem.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 44.Eyvazi S, Farajnia S, Dastmalchi S, Kanipour F, Zarredar H, Bandehpour M. Antibody based EpCAM targeted therapy of cancer, review and update. Curr Cancer Drug Targets. 2018;18((9)):857–68. doi: 10.2174/1568009618666180102102311. [DOI] [PubMed] [Google Scholar]
- 45.Bardia A, Messersmith WA, Kio EA, Berlin JD, Vahdat L, Masters GA, et al. Sacituzumab govitecan, a Trop-2-directed antibody-drug conjugate, for patients with epithelial cancer: final safety and efficacy results from the phase I/II IMMU-132-01 basket trial. Ann Oncol. 2021;32((6)):746–56. doi: 10.1016/j.annonc.2021.03.005. [DOI] [PubMed] [Google Scholar]
- 46.Husain AN, Colby TV, Ordonez NG, Allen TC, Attanoos RL, Beasley MB, et al. Guidelines for pathologic diagnosis of malignant mesothelioma 2017 update of the consensus statement from the international mesothelioma interest group. Arch Pathol Lab Med. 2018;142((1)):89–108. doi: 10.5858/arpa.2017-0124-RA. [DOI] [PubMed] [Google Scholar]
- 47.Zhao P, Yu HZ, Cai JH. Clinical investigation of TROP-2 as an independent biomarker and potential therapeutic target in colon cancer. Mol Med Rep. 2015;12((3)):4364–9. doi: 10.3892/mmr.2015.3900. [DOI] [PubMed] [Google Scholar]
- 48.Abdou AG, Shabaan M, Abdallha R, Nabil N. Diagnostic value of TROP-2 and CK19 expression in papillary thyroid carcinoma in both surgical and cytological specimens. Clin Pathol. 2019;12:2632010X19863047. doi: 10.1177/2632010X19863047. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data
Supplementary data
Data Availability Statement
Raw data are available upon reasonable request. All data relevant to the study are included in the article. All authors agree to be accountable for the content of the work.