Abstract
Adipose-derived stem or stromal cells (ASCs) possess promising potential in the fields of tissue engineering and regenerative medicine due to their secretory activity, their multilineage differentiation potential, their easy harvest, and their rich yield compared to other stem cell sources. After the first identification of ASCs in humans in 2001, the knowledge of their cell biology and cell characteristics have advanced, and respective therapeutic options were determined. Nowadays, ASC-based therapies are on the verge of translation into clinical practice. However, conflicting evidence emerged in recent years about the safety profile of ASC applications as they may induce tumor progression and invasion. Numerous in-vitro and in-vivo studies demonstrate a potential pro-oncogenic effect of ASCs on various cancer entities. This raises questions about the safety profile of ASCs and their broad handling and administration. However, these findings spark controversy as in clinical studies ASC application did not elevate tumor incidence rates, and other experimental studies reported an inhibitory effect of ASCs on different cancer cell types. This comprehensive review aims at providing up-to-date information about ASCs and cancer cell interactions, and their potential carcinogenesis and tumor tropism. The extracellular signaling activity of ASCs, the interaction of ASCs with the tumor microenvironment, and 3 major organ systems (the breast, the skin, and genitourinary system) will be presented with regard to cancer formation and progression.
Keywords: fat grafting, lipotransfer, adipose-derived stem or stromal cells, cytokines, secretome, exosomes, tumor cells, tumor microenvironment
Graphical Abstract
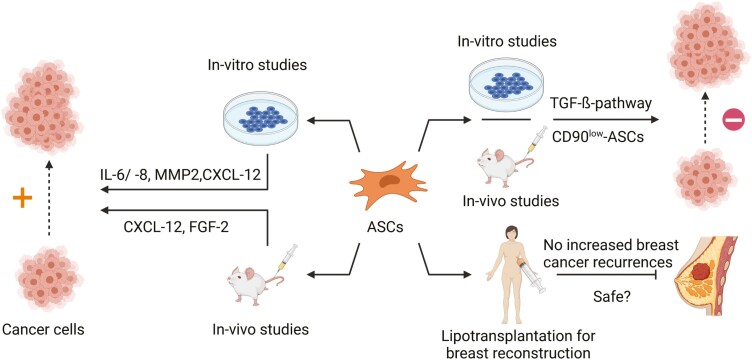
The oncogenic risk of ASCs sparks controversy. Numerous studies suggest a pro-oncogenic effect of ASCs on cancer cells. Contrariwise, other studies report an inhibitory and anti-oncogenic effect of ASCs, and clinical studies did not find elevated incidences of breast cancer recurrences after lipotransplantation. The divergent effects could be partially owed to the different behavior of ASCs in in-vitro and in-vivo studies versus clinical application as well as different reactions to various cancer entities.
Significance Statement.
We review the role of ASCs as promising stem cells for the treatment of various diseases versus their oncogenic risk after therapeutic application. Multiple in-vitro and in-vivo studies demonstrate pro- and anti-oncogenic effects of ASCs on different tumor entities. This study summarizes the effects of the ASCs’ extracellular signaling on cancer cells, their interactions with the tumor microenvironment, and the ASC oncogenic potential after transplantation into the 3 major organs. To comprehend the mechanism of ASC-mediated tumor formation and progression can help to advance the clinical utility of ASC-based therapies and to firmly establish ASC in routine clinical practice.
Introduction
Adipose-derived stem or stromal cells (ASCs) possess similar features as other adult stem cell types, including the ability to proliferate, the multilineage differentiation potential, and the secretion of a plethora of paracrine signaling factors and exosomes.1,2 They exhibit a variety of practical advantages over other forms of stem cells which sparked ample interest in ASCs and ASC-based therapies.1,2
Usage of embryonic stem cells (ESCs) or induced pluripotent stem cells (iPCS) is limited due to regulatory rules, ethical considerations as well as potential safety concerns.2 Grafting bone marrow-derived mesenchymal stem cells (BM-MSCs) or umbilical cord stem cells require invasive procedures, they are of lower number per milliliter of donor tissue, or they can only be obtained postpartum.2 Contrariwise, ASCs are easy to be harvested, and the often abundant adipose tissue/lipoaspirate commonly provided by liposuction or abdominoplasty operations contains large quantities of ASCs.2 These procedures yield around 0.5-0.7 × 106 stromal vascular cells/g adipose tissue and ASCs compromise up to 10% of those cells.3
ASCs are part of the so-called stromal vascular fraction (SVF) of adipose tissue, which consists of a variety of different cell lines, eg, vascular endothelial cells and their precursors, fibroblasts, pericytes, smooth muscle cells, lymphocytes, and ASCs.2-4 ASCs putatively express CD90, CD44, CD29, CD105, CD13, CD34, CD73, CD166, CD10, CD49e, and CD59 while being negative for CD31, CD45, CD14, CD11b, CD19, CD56, CD146, and HLA-DR.5 Furthermore, ASCs adhere to plastic surfaces and have multipotent differentiation capacity as they can differentiate toward the adipogenic, osteogenic, chondrogenic, and myogenic lineage.1,6
After harvesting adipose tissue, there are various methods to transplant or process the isolated graft. These methods vary regarding the final cell concentration (Fig. 1). Lipoaspirate is typically transplanted by either conventional autologous transplantation or via cell-assisted lipotransfer (CAL).7,8 In conventional autologous fat transplantation, the survival of the fat graft at the recipient site is limited as excessive amounts will likely cause volume loss and oil cyst formation.7,8 This prompted the development of CAL, since the augmented content of ASCs is considered to improve fat graft take rates compared to conventional lipotransplantation by enhancing neovascularization, which alleviates necrosis rates and fat graft loss.8 Moreover, transplantation of ASCs is thought to provide a natural pool for the regeneration of lost adipocytes.8 For CAL, enzymatic digestion and centrifugation alter the cellular composition to enrich the graft with ASCs. Additionally, ASCs can be isolated from the harvested adipose tissue and expanded on culture plates in vitro.9 After cultivation of a sufficient number of ASCs, they can be collected and transplanted for the targeted usage.
Figure 1.
Overview of the possible transplantation procedures of ASCs and potential therapeutic applications; harvested adipose tissue can be directly transplanted, digested, and centrifuged to enrich the graft with ASCs (CAL) or ASCs can be isolated, cultured for expenditure, and then transplanted, potential therapeutic applications for direct fat grafting and CAL are, eg, skin disorders or breast reconstruction; ex-vivo expanded ASCs are used, eg, for bone and nerve regeneration and in the field of tissue engineering. Abbreviations: ASCs, adipose-derived stem or stromal cells; CAL, cell-assisted lipotransfer.
ASC-based therapies show promising results in the field of tissue engineering10 and for a spectrum of different diseases ranging from contour deficits in the field of esthetic8,11 and regenerative medicine,4 bone,12,13 and cartilage regeneration,14,15 as well as amelioration of chronic wounds, eg, Crohn’s fistula-in-ano.16
However, with the emergence of potential oncogenic effects of ASCs,17-21 the safety profile of ASC was taken into question, when the cells are used for clinical applications. Clinical results advocating the safety of lipotransfer for breast augmentation,22-24 and in-vitro studies25,26 demonstrating an inhibitory effect of ASCs on tumor cells could not settle the concerns. That is one reason why the therapeutic application of ASCs still remains a twilight zone. Hence, deciphering the complex interactions of ASCs with tumor cells is crucial for assessing their oncogenic risk and thereby achieving maximum safety for patients. ASCs are not the sole MSC population linked with tumor-promoting properties as other sources of mesenchymal cells, eg, BM-MSCs, are also ascribed to create favorable conditions for tumor progression.27,28
In the following sections, we will outline the current knowledge of the ASC-derived extracellular signaling with perspective on potential alterations by as well as impacts on tumor cells, the crosstalk of ASCs with the tumor microenvironment (TME) as well as the relationship between ASCs and 3 major organs/organ systems, ie, the skin, the breast, and the genitourinary system.
Extracellular Signaling of ASCs
The various beneficial effects attributed to MSCs and ASCs most likely derive from their secretory activity,29,30 which shifted the research focus onto elucidating this extracellular signaling. This comprises the secretion of a broad repertoire of different soluble factors ranging from messenger RNA (mRNA) and microRNA (miRNA) to cytokines, chemokines, and growth factors.31,32 Exosomes likewise play a crucial role in conferring the ASCs’ paracrine signaling effects.33 A considerable portion of research regarding the ASC secretory activity explored the beneficial effects of adipose-derived extracellular vesicles (EVs) trafficking in myocardial ischemic injuries as well as neurological disorders, eg, Alzheimer’s disease, amyotrophic lateral sclerosis,33 or peripheral nerve regeneration.34 As the ASC effects are mostly mediated by extracellular signaling, it is possible that the pro- as well as anti-oncogenic effects of ASCs could primarily depend on their secretory activity. To comprehend which soluble factors secreted by ASCs foster tumor growth and metastasis susceptibility could help to reach a greater understanding of ASCs potential pro-oncogenic effects and may advance the clinical utility of ASC-based therapies. However, the current knowledge of how the paracrine signaling of ASCs is transformed by malignant tumors/tumor cells is limited.
The composition and concentrations of soluble factors are important mediators of tumor progression and invasion.35 Up to 474 different proteins are secreted by ASCs,36 yet 68 proteins were the most commonly synthesized in the proteome throughout multiple studies.32 Inflammatory stimulation of ASCs by exposure to TNF-α,37 a key regulator of tumor signaling38 and inducer of growth factors like vascular endothelial growth factor (VEGF) in ASCs,39 triggers the upregulation of signaling factors and proteases associated with detrimental effects in tumor settings like interleukin-6 (IL-6), interleukin-8 (IL-8), and matrix metalloproteases (MMPs)40-42 (see Table 1 for a synopsis of ASCs-derived soluble factors). ASCs secrete IL-6 and IL-843 and both cytokines exert important effects on tumor cells. In co-culture of ASCs with the breast cancer cell line 4T1 and colon cancer cell line CT26-cells, IL-6 regulates tumor-initiating properties, such as sphere generation and regulation of gene activity related to proliferation, eg, MKI67 and PCNA.44 Additionally, IL-6 and IL-8 foster invasion of breast cancer epithelial cells,45 and ASCs upregulate the expression of the aforementioned cytokines in melanoma and squamous carcinoma cells.46,47 Soluble MMPs, especially MMP-2 and MMP-9 are crucial for metastasis and angiogenesis by degradation of the vascular basal membrane, which promotes migration of endothelial cells and facilitates tumor metastasis.42 ASC-mediated upregulation of MMPs has been demonstrated in various cancer entities, eg, osteosarcoma, breast cancer, melanoma, squamous cell carcinoma (SCC), and ovarian cancer.45-49
Table 1.
Overview of ASC-mediated direct and indirect effects on various soluble signaling factors and their influence on cancer cells.
| Signaling factor | Cell type | Effect | |
|---|---|---|---|
| Interleukins | IL-6 | Breast and colon cancer cells | Expression of IL-6 of ASCs in co-culture ↑44 |
| Sphere generation of breast and colon cancer cells in co-culture with ASCs ↑44 | |||
| Upregulation of genes associated with proliferation, eg, MKI67 and PCNA in breast and colon cancer cells44 | |||
| Upregulation of cancer stem cell markers, eg, SOX2 and NANOG in breast and colon cancer cells44 | |||
| Normal and tumor mammary epithelial cells | Invasion of epithelial cells ↑45 | ||
| Expression of IL-6 in co-culture of melanoma and squamous carcinoma cells with ASCs ↑46,47 | |||
| IL-8 | Normal and tumor mammary epithelial cells | Invasion of epithelial cells ↑45 | |
| Expression of IL-8 in co-culture of melanoma and squamous carcinoma cells with ASCs ↑46,47 | |||
| MMPs | Osteosarcoma cells | MMP-2 and MMP-9 expression ↑ in osteosarcoma cells in co-culture with ASCs48 | |
| Normal mammary epithelial cells | MMP-2/-3/-7 and MMP-14 expression ↑ in mammary epithelial cells in co-culture with ASCs45 | ||
| Melanoma cancer cells | MMP-1/-2/-3 and MMP-10 expression ↑ in co-culture of melanoma cancer cells with ASCs46 | ||
| Squamous cell carcinoma | MMP-1/-3/-9 and MMP-10 expression ↑ in co-culture of squamous cell carcinoma cells with ASCs47 | ||
| Epithelial ovarian cancer cells | MMP-2 and MMP-9 expression ↑ after co-culture with ASCs49 | ||
| Chemokines | Prostate cancer cells | ASC migration to the tumor via the CXCL12/CXCR4 axis21 | |
| Melanoma cancer cells | Expression of CXCL12/13 and CCL2 ↑ in co-culture of melanoma cells with ASCs46 | ||
| Squamous cell carcinoma | Expression of CCL2 and CCL4 ↑ in co-culture of squamous carcinoma cells with ASCs47 | ||
Note: This table summarizes the effects of various soluble factors which are secreted by ASCs and explains their effects on cancer cells.
Abbreviations: ASCs, adipose-derived stem or stromal cells; IL-6, interleukin-6; IL-8, interleukin-8; MMPs, matrix metalloproteinases.
Adipokines are cytokines derived from developing and mature adipocytes, and aberrant adipokine signaling contributes to obesity-mediated cancer formation.50,51 Two essential adipokines are adiponectin and leptin, which might demonstrate reciprocal effects on the genesis of cancers. Adiponectin is generally attributed with a protective role in breast cancer, as low adiponectin levels are associated with an enhanced risk to develop breast cancer as well as more aggressive breast cancer phenotypes.50,51 However, the role of adiponectin might be more complex, since also pro-tumorigenic effects have been reported.50 Contrariwise, leptin has been ascribed a crucial role in the promotion of breast cancer.51 Suppression of autophagy, activation of signaling pathways associated with proliferation, regulation of numerous pro-angiogenic and pro-tumorigenic signaling factors, such as VEGF, induction of epithelial-to-mesenchymal transition (EMT), and stimulation of reactive oxygen species production, have been linked to leptin signaling.51 Hypoxia is commonly found in the tumorigenic microenvironment and induces a profound upregulation of leptin in ASCs.52 In obesity-associated ASCs, altered leptin signaling promotes tumor progress, metastasis, and radiation resistance in estrogen receptor-positive and triple-negative breast cancer cells.53-56 Thus, obesity-arbitrated altered adipokine signaling and elevated leptin secretion induced by hypoxic impact could constitute important mediators of ASCs’ pro-tumorigenic effects.
Chemokines regulate cell migration and interaction, which is essential in tumors to facilitate cancer growth, metastasis, and angiogenesis.57 Numerous studies demonstrate a chemokine-mediated effect on cancer formation and progression.57 In a prostate cancer model, ASCs homed and engrafted at the tumor site which was arbitrated by the CXCL12/CXCR4 axis.21 The co-culture of ASCs with melanoma cells and squamous carcinoma cells upregulates the expression of chemokines, eg, CXCL12/13 and CCL2,46 and of CCL2 and CCL4.47 Although the effect of increased chemokine expression was not further elucidated, the enhanced expression of chemokines suggests a pro-oncogenic risk, which could foster cancer proliferation and metastasis. Taken together, ASCs regulate multiple signaling factors in cancer cells which could facilitate ASCs’ pro-oncogenic effects, but the signaling factors’ exact effects are not well defined.
Some studies demonstrated that ASC-conditioned medium (CM) is capable of modulating tumor expansion and invasion.45,58,59 CM exerts either pro-oncogenic as well as opposing, inhibitory effects on cancer cell growth. In PC3M-luc2 cells, for instance, CM inhibits proliferation and induces apoptosis.60 Mechanistically, this is also conducted by miR-145, a known tumor suppressor.61 Admittedly, the heterogeneity of ASCs could account for these opposing effects.62 Li et al demonstrated that EVs derived from ASC subsets induce different effects on breast cancer cells.63 They isolated CD90high ASCs and transformed them into CD90low cells. EVs from these cells diminish tumor growth of the breast cancer cell lines E0771 and 4T1, when compared to CD90high ASCs. The underlying mechanisms remain to be investigated, but this study demonstrates that the altered EVs are at least partially responsible for the given effect. The inhibitory effect of CD90low ASC-derived EVs could be enhanced in vitro and in vivo by the addition of the anti-oncogenic miR-16-5p.64 Another comparative study examined whether tumor diseases influence the composition of ASC-derived exosomes. ASCs isolated from abdominal tissue of cancer patients with urological neoplasms versus cells of age-matched healthy donors do not differ in miRNA contents. Additionally, the ASCs exhibit similar growth kinetics, differentiation capacity, and molecular karyotyping.65 Therefore, the authors excluded an increased oncogenic potential of ASCs from tumor patients. For hepatocellular carcinoma cells (HCC), Ko et al demonstrated that ASCs-derived exosomes reduce HCC growth and elicit natural killer T (NKT)-cell enrichment in the tumor.66 There is ongoing scientific research in exploiting exosomes as biological carriers for cancer therapy.67 Transfection of ASC exosomes with miR-122, a known regulator of HCC chemosensitivity, in combination with sorafenib, an oral multikinase inhibitor drug for cancer treatment, renders the HCC cells more susceptible for the chemotherapy and promotes the efficacy of the sorafenib treatment.68 In osteosarcoma, however, ASC-derived exosomes trigger osteosarcoma cell invasion, migration, and proliferation in-vitro by inducing COLGALT2, vimentin, and MMP-2/-9 expression.48
Current data suggest a pivotal role of ASC extracellular signaling in the regulation of tumor expansion and vitality by showing pro- and anti-oncogenic effects in numerous tumor entities. Current knowledge is limited and further research is required to elucidate the molecular background for these findings.
ASCs and the Tumor Microenvironment
The TME is a complex and constantly evolving milieu harboring and accommodating tumor cells and shaping and redirecting encompassing cells toward a pro-tumorigenic behavior. The physiologic tissue homeostasis is disrupted and the surrounding tissue profoundly altered to facilitate tumor progression.69 It compromises numerous cell types, eg, stromal and endothelial cells, adipocytes, fibroblasts, and innate and adaptive immune cells in addition to neoplastic cells.69 Currently, the complex interactions between the TME and ASCs are rarely understood, but deciphering the crosstalk between ASCs and the TME could provide new insights into the oncogenic potential of ASCs and might unveil new mechanisms for therapeutic options.70
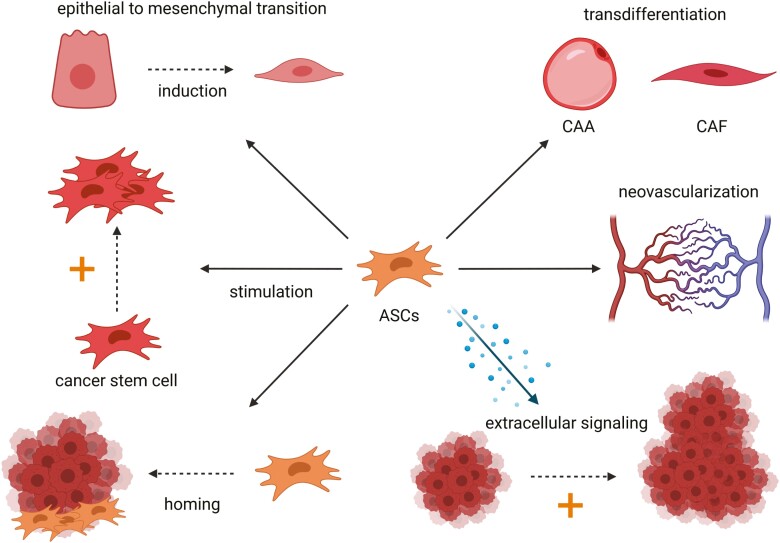
Two distinct cell subtypes in the TME, namely the cancer-associated adipocytes (CAAs) and the cancer-associated fibroblasts (CAFs), were extensively characterized and ASCs could be their natural precursors.71 Adipocytes secrete numerous factors, which modulate the nearby tissue and exert local and systematical effects.72 In the TME of breast cancer, adipocytes are crucial for the maintenance of the tumor homeostasis, as they secrete various factors which sustain tumor formation, survival, and progression.71 The adjacent adipocytes are coined CAAs and distinguish themselves from normal adipocytes through altered phenotypic appearance. They are characterized by a smaller, more irregular shape and a modified secretome with an elevated concentration of inflammatory cytokines and proteases.71 CAA-derived adipokines like leptin, IL-6, CCL2, and CCL5 drive the tumor expansion, tumor migration, and invasiveness.71 Transdifferentiation of ASCs toward CAAs could be a potential mechanism for the CAA generation and their malignant tropism in the TME (Fig. 2).
Figure 2.
Potential pro-oncogenic mechanisms of ASCs are depicted in this figure. ASCs may induce epithelial-to-mesenchymal transition (EMT), stimulate cancer stem cell growth/tumor growth, transdifferentiate toward CAA/CAF, enhance neovascularization of the tumor, and are capable of migration, the so-called homing, to the tumor. Abbreviations: ASCs, adipose-derived stem or stromal cells; CAA, cancer-associated adipocytes; CAF, cancer-associated fibroblasts.
Like CAAs, CAFs play a pivotal role in the TME and are distinct from normal fibroblasts.73 Orchestrating the alteration of the extracellular matrix (ECM) is a key and complex activity of CAFs. They are capable of increasing rigidity of the ECM by producing matrix crosslinking enzymes as well as remodeling the ECM to generate pathways for cancer cell migration.73 Increased stiffness and alteration of the ECM promotes tumor expansion by triggering proliferation and invasion, by attenuating blood flow, which can select more cancer cells, and by hampering immunosurveillance which restricts motility of T cells.74,75 Moreover, CAFs secrete a plethora of growth factors, cytokines, and exosomes which enhance tumor growth, invasiveness, and steer angiogenesis. CAFs are abundantly present in the TME, yet their cellular origin is unclear. Most likely, CAFs originate from local fibroblasts or mature adipocytes.76-78 ASCs likewise demonstrate plasticity toward CAF differentiation and could constitute a pristine stock for the generation of CAFs.18,79,80 Admittedly, ASCs modify the ECM components through secretion of various collagens, elastin, and fibronectin.81 By paracrine and juxtacrine signaling, ASCs align and organize surrounding cells in their close vicinity.81 In a xenograft cancer model of human pancreatic ductal adenocarcinoma, ASCs significantly contribute to a stroma-rich microenvironment surrounding the cancer cell harboring ductal glands, which notably promotes tumor growth. Additionally, different CAF subpopulations were generated by the transdifferentiation of ASCs.80 Obesity also affects ASCs’ modulation of the ECM.82 Obesity-associated ASCs partially unfold ECM deposits and thereby increase rigidity of the ECM. In turn, tumor growth of MDA-MB-231 human breast cancer cells is stimulated by these obesity-associated processes.82 In conclusion, ASCs can promote ECM remodeling and rigidity, and CAF can be generated by transdifferentiation of ASCs, which in turn could enhance the transformation of ECM toward a pro-tumorigenic microenvironment.
Angiogenesis is critical for cancer development and metastasis, and ASCs stimulate the expression of key pro-angiogenic factors like VEGF and foster angiogenesis in tumors.46,47,83 In prostate tumors, ASCs enhance angiogenesis by elevated fibroblast growth factor 2 (FGF2) expression with increased capillary density of tumors.21 In cervical cancer, ASCs stimulate the generation of vascularized tumors.84 Thus, ASCs can support angiogenesis in growing tumors, which can lead to more advanced and disseminated cancers.
ASCs like BM-MSCs exhibit the natural capacity to migrate—the so-called homing—to the tumor site.85,86 Homing is a complex and multistep process, and numerous signaling molecules, receptors, and proteins are involved in this process. The CM of breast cancer cell lines (MDA-MB-231 and 4T1) elicits ASC migration via the platelet-derived growth factor (PDGF)-BB/PDGFR-β signaling pathway.87 Zhang et al demonstrated in various rodent tumor models that ASCs are specifically attracted to distant tumors, engraft at the tumor site, and facilitate tumor expansion.85 Moreover, organs with filtering properties like the lung, the liver, and spleen do not aggregate ASCs so that tumor tissue seems to be the specific attractant for ASCs.85 Therefore, application of ASCs cannot only be appreciated as a local therapy and homing of ASCs to the cancer site must be considered when administering ASCs distantly. Moreover, distant homing of circulating ASCs could be fostered at tumor sites, which naturally lack adjacent adipose tissue as tumors preferentially recruit progenitor cells from local adipose tissue. In paucity of adipose tissue and stem cells in their close vicinity, tumors draft ASCs or BM-MSCs from more distant sources.88 Additionally, the abundance of circulating mesenchymal progenitor cells in the peripheral blood is significantly increased in obese and colorectal cancer patients.89 Thus, known cancer woes in addition to obesity could facilitate homing of ASCs. The mechanism of MSCs/ASCs homing has been exploited in various studies, which aimed at tailoring targeted therapies for the treatment of tumors.70 Genetically modified MSCs, which produce anti-cancerous biological agents, successfully home to the tumor site in a murine lung metastatic cancer model, reduce the tumor size, and clear metastases.90
EMT is a relevant process in the genesis of tumors and metastasis. Epithelial cells gradually transform into a phenotypic state distributed along the epithelial-to-mesenchymal spectrum and lose their epithelial fate and polarity.91 It is a dynamic and complex mechanism that involves numerous transcription factors, proteins, and signaling pathways.91 The transformed cell types are associated with increased stem cell-like features, invasion, tumor heterogeneity, and therapeutic resistance and can drive tumor development and progression.91 EMT and EMT-like induction elicited by ASCs has been demonstrated in distinct tumor cell types, eg, breast cancer cells, lung cancer cells, and glioma cells.45,92-94 Besides EMT, ASCs interact with cancer stem cells (CSCs).44 CSCs are a subpopulation of cells, which are attributed to stem cell-like features like self-renewal and differentiation capacity toward more differentiated cancer cell states.95 They are thought to be responsible for the formation, progression, and relapse of cancers.95 ASCs can enhance the sphere generation of breast and colon cancer cells, increase the expression of CSC-associated markers, and drive tumor initiation in distinct tumor cell types.44,95 EMT and the interaction of ASCs with CSCs constitute further mechanisms by which ASCs could steer the promotion and progression of tumor formation.
ASCs possess great immunomodulatory capacity, which highlights their potential in the treatment of autoimmune and inflammatory pathologies.96 They express low levels of MHC-I classes and no major MHC-II classes, which renders them hypoimmunogenic and immune-privileged so that they can evade the immune response.96 Their immunomodulatory competence is based on various mechanisms, such as the secretion of immune-regulatory cytokines, the inhibition of B-cell maturation and antibody secretion, and interaction with numerous classes of T cells.96 Factors in the cancerous microenvironment like hypoxia,97 alterations in the ECM constitution82 or increased levels of pro-inflammatory cytokines,57 alter ASCs and could skew ASCs toward an immunosuppressive phenotype. Inflamed adipose tissue in tumors recruits numerous immunosuppressive cells, eg, myeloid-derived suppressor cells, tumor-associated macrophages, and T-regulatory cells.98 Hints for a direct involvement of ASCs to create an immunosuppressive microenvironment in tumors have also been confirmed. CM of breast cancer-derived ASCs upregulates anti-inflammatory cytokine secretion, such as IL-4, TGF-β1, and IL-10, in peripheral blood lymphocytes.99 Cancer-derived ASCs may additionally regulate T-regulatory cells as the number of CD4+ CD25high Foxp3+ T-regulatory cells were increased in the presence of cancer-derived ASCs or their CM.99,100 CD4+ CD25high Foxp3+ T cells are responsible for the inhibition of infiltrating CD4+, CD25−, and CD8+ T cells.99,101 For a further review of the interactions of ASCs with immune cells in the context of cancer, see Table 2.102-107 The immunomodulatory properties of ASCs in non-cancer settings have also been extensively reviewed recently.96,108 Regarding the role of the immune system in combating cancer, the immunosuppressive effects of ASCs could contribute to the genesis and/or progression of tumors.
Table 2.
Synopsis of the interactions of ASCs with immune cells in cancer and non-cancer settings.
| Immune cell type | ASC-mediated effect | Source |
|---|---|---|
| Peripheral blood lymphocyte (PBL) | Breast cancer-derived ASCs’ CM upregulates anti-inflammatory cytokines IL-4, TGF-β1, and IL-10 and increase CD4+, CD25high Foxp3+ T-regulatory cells in PBL | 97 |
| Breast cancer-derived ASCs’ CM upregulate IL-10, TGF-β, and PGE2 synthesis in PBL and stimulate PBL proliferation | 100 | |
| Normal and breast cancer-derived ASCs reduce NKG2D+ and CD69+ natural killer cell subpopulations in PBL in indirect culture | 102 | |
| ASCs induce increased apoptosis, but diminished necrosis in PBL in co-culture | 104 | |
| Naïve CD4+ T cells | Breast cancer-derived ASCs induce an increase in production of IL-10 and TGF-β and expand CD4+CD25+ FOXP3+Helios+, CD4+CD25− FOXP3+Helios+, and CD25+ FOXP3+CD73+CD39+ regulatory T cells in co-culture | 98,101 |
| B-lymphocytes | Proliferation of B-lymphocytes from tumor-draining lymphocytes is significantly inhibited by ASCs and the TNF-α+/IL-10+ B cells ratio is significantly diminished by breast cancer-derived ASCs in co-culture | 103 |
| Macrophages | Obesity-associated ASCs alter macrophages toward a pro-tumoral phenotype and upregulate genes related to inflammation, angiogenesis, immune suppression, invasiveness, tumor growth, and metastasis | 105 |
Note: The interactions of ASCs with immune cells in non-cancer and cancer settings are summarized in Table 2.
Abbreviations: ASCs, adipose-derived stem or stromal cells; CM, conditioned medium.
In summary, the interactions of ASCs with the TME are still poorly understood, but the current knowledge nonetheless demands caution as multiple potential pro-oncogenic mechanisms of ASCs have been demonstrated. ASCs can remotely home to the cancer site, mediate tumor expansion, EMT, and interact with CSCs and immunomodulatory cells, potentially creating an immunosuppressive environment. Moreover, they can transdifferentiate to CAFs and likely CAAs, thereby bolstering the stock of CAFs and CAAs.
SVF-Mediated Effects on Cancer Cells
As mentioned earlier, the SVF is a heterogeneous mixture of a range of different cell populations, and ASCs comprise around 10% of these cells.3,4 Application of the SVF could be superior to the sole application of ASCs due to numerous practical advantages.109 The requirement for ex-vivo expansion of ASCs is costly and requires further regulatory steps. On the other hand, SVF-based therapies are performed single-stage during surgery and the paracrine signaling of the various cell types contained in the SVF alongside ASCs could be beneficial over the exclusive administration of ASCs.109 The SVF secretes a significantly higher amount of various soluble signaling factors like the angiogenic IL-8, macrophage inflammatory protein 1α and 1β and attenuated levels of pro-inflammatory cytokines IFN-γ and IL-12 when compared to ASCs.110 Contrariwise, VEGF, IL-7, and anti-inflammatory cytokines, such as IL-10 and IL-13, are released at higher levels from ASCs. Nevertheless, the application of the SVF can constitute a similar pro-oncogenic risk as supposed for ASCs. However, there is only a limited number of studies that address this issue. Lee et al examined the effects of SVF injection in proximity to cancer cells (MDA-MB-231, a human-derived breast cancer cell line) in a breast cancer xenograft model in NOD/SCID mice and found no tumor-promoting effect of the SVF.111 Two clinical studies investigated the effects of SVF-augmented fat grafts for breast reconstruction after breast cancer surgery and observed no increased breast cancer recurrence to the matched control group over a period of 3 and 5 years.112,113 Locoregional cancer recurrences were 3.7% and 2.4% in the SVF-treated patients compared to 4.13% and 1.6% in the untreated control group.112,113 Importantly, a direct comparison between the cancerous potential of SVF versus ASCs has not been performed, yet. Theoretically, a significant oncogenic difference between ASC- and SVF-based therapy could exist which could be due to direct cell-cell interactions between different populations in the SVF or divergent paracrine signaling. Thus, further research is warranted to elucidate the potential tumorigenic effects of the SVF and to determine specific differences between ASC and SVF applications.
In the enclosing sections, we want to illustrate the interactions and effects of ASCs in clinical settings, when the cells are already applied, and therapies are feasible. Additionally, we chose the group of urogenital cancers as a representative group to demonstrate the mechanism by how ASC could elicit cancer progression in solid organ tissues.
ASC-Mediated Effects on Breast Cancer
As breast cancer is the most frequent tumor in women, and the standard treatment commonly involves the excision of breast volume to a varying degree, there is a major demand for breast reconstruction.114 Lipofilling of the breast represents an easy and elegant method to treat volume deficits. However, multiple studies found concerning evidence of ASCs promoting favorable conditions for breast cancer cells in vitro. Thus, the application of ASCs could lead to cancer relapse, especially regarding cases with residual cancer cells after surgery. Co-culture of ASCs with different human breast cancer cell lines and primary breast cancer cells concomitantly upregulate multiple tumor-associated genes and their corresponding proteins like IL-6/-8 and a range of different MMPs, which are associated with favorable effects for cancer progression.115 Moreover, the ASCs’ extracellular signaling exerts concerning effects on normal mammary and carcinoma cells.45,58 Kengelbach-Weigand et al showed that the ASCs’ secretory activity enhances proliferation, transmigration, and the invasiveness of normal mammary epithelial cells and invasive inflammatory ductal carcinoma cells.45,58 The role of IL-6 and IL-8 as negative regulators of normal and tumor breast epithelial cells stimulate the invasiveness of these cells.45 ASCs derived from both healthy and cancer patient’s tumor-adjacent and tumor non-adjacent adipose tissue foster the proliferation of the human breast cancer cell line MCF-7.25 In a murine breast cancer xenograft model of triple-negative human breast cancer (MDA-MB-231 cells), ASCs promote tumor growth and metastasis and altered expression of vimentin (EMT marker), MMP-9, VEGF, and IL-8 within the tumor.116 Furthermore, ASCs isolated from tumorous breast tissue are permanently altered in comparison to ASCs from healthy donors as the ASCs from tumor patients are marked by distorted gene expression, eg, downregulated GDF5, GDF6, IGF1, PDGFRB, and TGFB3 expression, and overall reduced cytokine release.20 In addition, they exhibit higher migratory activity and stimulate tumor proliferation stronger than their healthy counterparts.20 Mechanistically, multiple signaling pathways are involved in the promotion of breast cancer cell proliferation and migration such as the epidermal growth factor (EGF)/EGF-receptor/Akt-, the hepatocyte growth factor (HGF)/c-Met-, the insulin-like growth factor (IGF) insulin-like growth factor binding protein (IGFBP)-, the phosphorylated c-Kit/mitogen-activated protein kinase (MAPK)-p38/E2F-transcription factor 1 (E2F1), and the Wnt signaling pathways.17,117-120
In contrast to these findings, some in-vitro studies demonstrated an inhibitory effect of ASCs or the ASC’s extracellular signaling on breast cancer cells but pro-oncogenic activities of ASCs were also reported within the same studies. Human SKBR3 breast cancer cells, either co-cultured with ASCs or subjected to ASC-derived CM proliferate less, and they expand their sensitivity to chemotherapy.121 Nonetheless, in the same study ASCs and their CM transformed the morphology of the human breast cancer cell line SKBR3 and triggered the EMT, which is linked to increased invasion as well as the promotion of tumor progression.91 In another study, the ASC-derived CM exerts an anti-proliferative effect on the human breast cancer cell line MCF-7 in a concentration-dependent manner whereas co-culture of ASCs with MCF-7 supports the cancer cell proliferation.25 In addition to these findings, there is also a major discrepancy between the in-vitro results and the clinical situation. In a variety of different clinical studies22-24,122-129 (see Table 3 for a synopsis of the study designs), no augmented locoregional or systemic recurrence happened after fat grafting except for one study, which found an increased risk for local recurrence when fat grafting is conducted in close temporal succession after mastectomy as well as 2 unexpected cases of inflammatory breast cancer recurrences.23 However, the authors expressed their caution in drawing conclusions of the increased local recurrence rates due to potential biases, and they concluded lipofilling of the breast to be a safe procedure. Regarding local recurrence after application of fat grafting, a highly alarming and unexpected case of osteosarcoma recurrence in temporal context after autologous fat grafting has been reported.130 In this case, there was a late local recurrence 13 years after the initial osteosarcoma occurrence and in close temporal succession of 18 months after lipotransplantation at the original tumor site.130 Local cancer relapse of osteosarcoma after such an extended time period is not anticipated and in-vitro and in-vivo data suggest fat grafting to be a risk factor for osteosarcoma progression.130
Table 3.
Overview of the studies which examined fat grafting for breast reconstruction after breast oncological surgery with regard to oncological safety.
| Number of patients | Study design | Mean follow-up | Oncological outcome | Source |
|---|---|---|---|---|
| 719 cases, 670 controls, 305 cancer-free breasts with lipofilling procedure | Matched controlled study | 60 months, 44 months, and 73 months, respectively | No increased locoregional or systematic recurrence, no secondary breast cancer | 24 |
| 322 cases, 322 controls | Matched case-control study | 4.6 years | No increased locoregional, systematic, or contralateral tumor recurrence, no increased axillary node metastasis | 122 |
| 248 cases, 581 controls | Retrospective chart review | 45.6 months for cases and 38.8 months for controls | No increased local or systematic recurrences | 123 |
| 167 fat grafting procedures in 108 patients | Retrospective case series | 20.2 months | No reported locoregional cancer recurrence | 124 |
| 225 cases of breast cancer recurrences, 972 of patients without recurrence | Multicenter case-cohort study | NA | No adjusted increased risk for local-regional or systematic cancer recurrences after fat grafting | 125 |
| 68 fat grafting procedures in 67 patients | Multicenter, prospective, single-arm clinical trial | 12 months | No reported locoregional cancer recurrence | 126 |
| 646 fat grafting procedures in 513 patients | Retrospective case series | 19.2 months | No control group, slightly increased locoregional recurrence rate when compared to the literature | 127 |
| 205 cases, 410 controls | Retrospective case-control study | 40.4 months for cases, NA for controls | No increased regional or distant recurrences, but increased locoregional recurrence in lipofilling group in close temporal succession after surgery, 2 unexpected cases of inflammatory breast cancer recurrence in lipofilling group | 23 |
| 72 cases, 72 controls | Retrospective case-control study | 66.8 months for cases, 61.9 months for controls | No increased locoregional or systematic cancer recurrences | 128 |
| 93 cases, 93 controls | Retrospective matched cohort analysis | 48.7 months for cases, 49.9 months for controls | No increased locoregional recurrences or divergent 5-year survival | 129 |
Note: This table summarizes the studies which examined fat grafting for breast reconstruction following mastectomy/breast cancer excision with regard to oncological safety.
Abbreviation: NA, not available.
In conclusion, the current scientific evidence of the relationship between ASCs and breast cells is inconsistent and at times contradictory. Experimental data suggest an oncogenic risk for the application of ASC which, however, does not seem to translate to current clinical findings.
ASC-Mediated Effects on Urogenital Cancer
Obesity is a risk factor for prostate and cervical cancer development and to a weaker extent for ovarian cancer.131-133 In prostate cancer, higher incidence numbers of aggressive cancer and increased tumor size are reported in obese patients.134 Homing and engraftment of ASCs at the tumor site and ASC-mediated tumor progression could account for these phenomena. Thus, the interactions between ASCs and the cancer types could help to understand the underlying mechanism of obesity as a risk factor.
In a murine model of prostate cancer, the human PC3 prostate cancer cell line attracts distantly injected ASCs via the CXCL12/CXCR4 axis, which leads to engraftment of ASCs in the tumor tissue.21 Subsequently, ASCs stimulate tumor growth of these cells and steer angiogenesis by elevated FGF2 expression. A more recent study suggested reciprocal effects by demonstrating that ASCs are capable of hampering the growth of human prostate cancer cell lines, namely androgen-responsive (LNCaP) and androgen-nonresponsive (PC3) cells, and initiate apoptosis, presumably via the TGF-β pathway.26
In cervical cancer, ASCs seem to promote progression and induce a malignant phenotype. Castro-Oropeza et al examined the interaction between ASCs and numerous established human cervical cancer cell lines (HeLa, SiHa, and CaSki).84 ASCs distort the transcriptome and alter multiple cellular processes involved in cell motility, cell death, and cell communication. Interestingly, the EMT pathway is affected mostly, so ASCs may induce EMT in cervical cancer. Moreover, ASCs increase migration and invasion as well as angiogenesis and carcinogenesis, while the proliferation of these cell lines remains stable. Regarding proliferation and aggressiveness, Zhai et al demonstrated that ASCs elicit growth and invasion in cervical cancer by modulation of the HGF/c-Met pathway.135
In ovarian cancer, ASCs have a stimulating effect on cancer growth and invasion, which is mediated by elevated MMP-2 and MMP-9 expression, thymosin beta 4X-linked expression, and stabilization of the TAZ protein by PAX8 expression.49,136,137
In conclusion, ASCs seem to promote the progression of ovarian and cervical cancer whereas in prostate cancer the current data are less clear. The current data suggest that elevated abundance of ASCs in obesity could constitute a risk factor for the formation of the mentioned cancers as engraftment of ASCs at the tumor site by the mechanisms of homing is feasible.
ASC-Mediated Effects on Skin Cancer
CAL is regarded as a promising tool in the field of skin regenerative medicine and ASCs are attributed various beneficial effects in skin-based therapies, eg, amelioration of photoaging, wound healing, and skin diseases like atopic dermatitis.138-140 However, a few studies addressed the relationship between ASCs and the various skin cancer types although CAL is readily applied in areas susceptible to skin cancer formation.
Preisner et al demonstrated a bi-directional crosstalk between ASCs and melaoma cells in numerous established human melanoma cell lines (G-361, SK-Mel-5, MeWo and A2058) as well as in primary melanoma cells.46 Co-culturing enhances proliferative activity in primary melanoma cells, induces upregulation of tumor-associated genes, ie, CXCL12, PTGS2, IL-6, IL-8, MMP-2, and HGF, and mediates upregulation of tumor-promoting proteins, eg, CCL2, IL-6, IL-8, VEGF, and MMP-2.46 Moreover, the migratory and invasion capacity of ASCs and melanoma cells are enhanced when both cell types are co-cultured.46 Interestingly, changes in protein expression like IL-6, VEGF, and MMP-1/-2/-3 were more apparent in melanoma cells than in ASCs.46 Contrariwise, one study reported an inhibitory effect of ASC CM on human melanoma cell lines A375SM and A375P with an increase of cell number in the G0/G1 phase of the cell cycle and elevated apoptosis rates.141 Additionally, ASCs administered in a xenograft melanoma mouse model are specifically attracted to the tumor and reduce tumor growth.141 In an approach to design a targeted immunomodulatory therapy for melanoma, genetically modified ASCs, specifically designed to express elevated doses of IL-2, diminish apoptosis rate of murine B16F10 melanoma cells in vitro and support tumor growth in vivo.70 Vice versa, non-modified ASCs do not alter tumor progression nor apoptosis rate.
Another study investigated the crosstalk between ASCs and SCC.47 Co-culture of ASCs with primary human SCC and A-431-SCC (human SCC cell line) increases the invasiveness of both cancer cell types. It upregulates pro-angiogenic proteins like FGF2, IL-8, VEGF, CCL2, and CXCL6 in ASCs and SCC, as well as inflammatory markers like IL-6 and MMP-1/-2/-3. These data suggest an enhanced oncogenic risk with promotion of angiogenesis and metastasis, if ASCs are brought in contact with SCC.
Taken together, the reviewed data indicate that ASCs are capable of promoting SCC and melanoma tumor expansion as well as the upregulation of tumor-associated proteins and the invasiveness of distinct tumor cell lines. Regarding melanoma, reciprocal results suggest that ASCs can likewise exert an anti-oncogenic effect on melanoma cells. Nonetheless, caution must be exercised administering ASCs in areas prone to the formation of skin cancers and in the proximity of suspicious skin lesions.
Conclusion
ASC-based therapies constitute a practical and feasible possibility toward stem cell-based approaches in the clinical setting. Ample in-vitro studies demonstrate ASC-mediated tumor cell expansion and invasiveness in numerous distinct tumor cell lines. However, the available data spark controversy as clinical studies do not report elevated tumor incidence/recurrence rates after the application of ASC therapies. Also, some studies report of ASCs inhibiting tumor growth. The underlying reasons could be partially due to differential behavior of ASCs in in-vitro versus in-vivo experiments, differential reactions of ASCs toward multiple tumor entities and in different species and divergent modulation of ASC through the tumor environment.
So, what are the implications for the clinical application of ASCs? Do preexisting tumor diseases prohibit ASC administration? What are the necessary diagnostic steps to be taken beforehand? Moreover, the question arises whether ASCs solely induce tumor tropism in preexisting pre-cancerous and cancer cells lines or whether ASCs can also drive the necessary malignant transformation process of normal cells toward neoplastic cells. Consequently, the existing data urge for further research and for caution toward the careless administration of ASCs, especially regarding tissue prone for carcinogenesis and in patients with known tumor occurrences as the actual risk of ASC application has yet to be determined. Thus, to monitor the long-term results and adverse effects of fat grafting for all esthetic and reconstructive surgical procedures, the Plastic Surgery Foundation (PSF) and the American Society of Plastic Surgeons (ASPS) set up a web-accessible database—General Registry for Autologous Fat Graft (GRAFT) (https://www.thepsf.org/research/registries/graft)—that prospectively collects all data regarding fat grafting. Additionally, there is an active clinical trial (https://clinicaltrials.gov/ct2/show/NCT02339779?cond=fat+grafting&draw=2&rank=73), which investigates the oncological safety of fat grafting for breast reconstruction in comparison to implant-based breast reconstruction. The ongoing research will help to provide new insights into the safety and efficacy of fat grafting and will be beneficial for patients and specialists alike.
Acknowledgments
Figures were created with BioRender.com.
Contributor Information
Vincent G J Guillaume, Department of Plastic Surgery, Hand Surgery, Burn Center, University Hospital RWTH Aachen, Aachen, Germany.
Tim Ruhl, Department of Plastic Surgery, Hand Surgery, Burn Center, University Hospital RWTH Aachen, Aachen, Germany.
Anja M Boos, Department of Plastic Surgery, Hand Surgery, Burn Center, University Hospital RWTH Aachen, Aachen, Germany.
Justus P Beier, Department of Plastic Surgery, Hand Surgery, Burn Center, University Hospital RWTH Aachen, Aachen, Germany.
Funding
None declared.
Conflict of Interest
The authors declared no potential conflicts of interest.
Author Contributions
V.G.J.G.: Conception and design, manuscript writing; T.R.: Conception and design, manuscript writing, final approval of manuscript; A.M.B.: Manuscript writing, final approval of manuscript; J.P.B.: Administrative support, manuscript writing, final approval of manuscript.
Data Availability
No new data were generated or analyzed in support of this research.
References
- 1. Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315-317. [DOI] [PubMed] [Google Scholar]
- 2. Ntege EH, Sunami H, Shimizu Y. Advances in regenerative therapy: a review of the literature and future directions. Regen Ther. 2020;14:136-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Oedayrajsingh-Varma MJ, van Ham SM, Knippenberg M, et al. Adipose tissue-derived mesenchymal stem cell yield and growth characteristics are affected by the tissue-harvesting procedure. Cytotherapy. 2006;8(2):166-177. [DOI] [PubMed] [Google Scholar]
- 4. Bora P, Majumdar AS. Adipose tissue-derived stromal vascular fraction in regenerative medicine: a brief review on biology and translation. Stem Cell Res Ther. 2017;8(1):145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mildmay-White A, Khan W. Cell surface markers on adipose-derived stem cells: a systematic review. Curr Stem Cell Res Ther. 2017;12(6):484-492. [DOI] [PubMed] [Google Scholar]
- 6. Mizuno H, Zuk PA, Zhu M, Lorenz HP, Benhaim P, Hedrick MH. Myogenic differentiation by human processed lipoaspirate cells. Plast Reconstr Surg. 2002;109(1):199-209; discussion 210. [DOI] [PubMed] [Google Scholar]
- 7. Simonacci F, Bertozzi N, Grieco MP, Raposio E. From liposuction to adipose-derived stem cells: indications and technique. Acta Biomed. 2019;90(2):197-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Matsumoto D, Sato K, Gonda K, et al. Cell-assisted lipotransfer: supportive use of human adipose-derived cells for soft tissue augmentation with lipoinjection. Tissue Eng. 2006;12(12):3375-3382. [DOI] [PubMed] [Google Scholar]
- 9. Patrikoski M, Mannerström B, Miettinen S. Perspectives for clinical translation of adipose stromal/stem cells. Stem Cells Int. 2019;2019:5858247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sterodimas A, de Faria J, Nicaretta B, Pitanguy I. Tissue engineering with adipose-derived stem cells (ADSCs): current and future applications. J Plast Reconstr Aesthet Surg. 2010;63(11):1886-1892. [DOI] [PubMed] [Google Scholar]
- 11. Xiong BJ, Tan QW, Chen YJ, et al. The effects of platelet-rich plasma and adipose-derived stem cells on neovascularization and fat graft survival. Aesthetic Plast Surg. 2018;42(1):1-8. [DOI] [PubMed] [Google Scholar]
- 12. Mende W, Gotzl R, Kubo Y, et al. The role of adipose stem cells in bone regeneration and bone tissue engineering. Cells. 2021;10(5):975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yoshida Y, Matsubara H, Fang X, et al. Adipose-derived stem cell sheets accelerate bone healing in rat femoral defects. PLoS One. 2019;14(3):e0214488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang J, Liu Y, Chen Y, et al. Adipose-derived stem cells: current applications and future directions in the regeneration of multiple tissues. Stem Cells Int. 2020;2020:8810813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pers YM, Rackwitz L, Ferreira R, et al. ; ADIPOA Consortium . Adipose mesenchymal stromal cell-based therapy for severe osteoarthritis of the knee: a phase I dose-escalation trial. Stem Cells Transl Med. 2016;5(7):847-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhou C, Li M, Zhang Y, et al. Autologous adipose-derived stem cells for the treatment of Crohn’s fistula-in-ano: an open-label, controlled trial. Stem Cell Res Ther. 2020;11(1):124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yan XL, Fu CJ, Chen L, et al. Mesenchymal stem cells from primary breast cancer tissue promote cancer proliferation and enhance mammosphere formation partially via EGF/EGFR/Akt pathway. Breast Cancer Res Treat. 2012;132(1):153-164. [DOI] [PubMed] [Google Scholar]
- 18. Jotzu C, Alt E, Welte G, et al. Adipose tissue-derived stem cells differentiate into carcinoma-associated fibroblast-like cells under the influence of tumor-derived factors. Anal Cell Pathol (Amst). 2010;33(2):61-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chapelin F, Khurana A, Moneeb M, et al. Tumor formation of adult stem cell transplants in rodent arthritic joints. Mol Imaging Biol. 2019;21(1):95-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Plava J, Cihova M, Burikova M, et al. Permanent pro-tumorigenic shift in adipose tissue-derived mesenchymal stromal cells induced by breast malignancy. Cells. 2020;9(2):480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lin G, Yang R, Banie L, et al. Effects of transplantation of adipose tissue-derived stem cells on prostate tumor. Prostate. 2010;70(10):1066-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Waked K, Colle J, Doornaert M, Cocquyt V, Blondeel P. Systematic review: the oncological safety of adipose fat transfer after breast cancer surgery. Breast. 2017;31:128-136. [DOI] [PubMed] [Google Scholar]
- 23. Silva-Vergara C, Fontdevila J, Weshahy O, Yuste M, Descarrega J, Grande L. Breast cancer recurrence is not increased with lipofilling reconstruction: a case-controlled study. Ann Plast Surg. 2017;79(3):243-248. [DOI] [PubMed] [Google Scholar]
- 24. Kronowitz SJ, Mandujano CC, Liu J, et al. Lipofilling of the breast does not increase the risk of recurrence of breast cancer: a matched controlled study. Plast Reconstr Surg. 2016;137(2):385-393. [DOI] [PubMed] [Google Scholar]
- 25. Trivanović D, Nikolić S, Krstić J, et al. Characteristics of human adipose mesenchymal stem cells isolated from healthy and cancer affected people and their interactions with human breast cancer cell line MCF-7 in vitro. Cell Biol Int. 2014;38(2):254-265. [DOI] [PubMed] [Google Scholar]
- 26. Takahara K, Ii M, Inamoto T, et al. Adipose-derived stromal cells inhibit prostate cancer cell proliferation inducing apoptosis. Biochem Biophys Res Commun. 2014;446(4):1102-1107. [DOI] [PubMed] [Google Scholar]
- 27. Papaccio F, Paino F, Regad T, Papaccio G, Desiderio V, Tirino V. Concise review: cancer cells, cancer stem cells, and mesenchymal stem cells: influence in cancer development. Stem Cells Transl Med. 2017;6(12):2115-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bergfeld SA, DeClerck YA. Bone marrow-derived mesenchymal stem cells and the tumor microenvironment. Cancer Metastasis Rev. 2010;29(2):249-261. [DOI] [PubMed] [Google Scholar]
- 29. Mitchell R, Mellows B, Sheard J, et al. Secretome of adipose-derived mesenchymal stem cells promotes skeletal muscle regeneration through synergistic action of extracellular vesicle cargo and soluble proteins. Stem Cell Res Ther. 2019;10(1):116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Giebel B, Kordelas L, Börger V. Clinical potential of mesenchymal stem/stromal cell-derived extracellular vesicles. Stem Cell Investig. 2017;4:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hong P, Yang H, Wu Y, Li K, Tang Z. The functions and clinical application potential of exosomes derived from adipose mesenchymal stem cells: a comprehensive review. Stem Cell Res Ther. 2019;10(1):242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kapur SK, Katz AJ. Review of the adipose derived stem cell secretome. Biochimie. 2013;95(12):2222-2228. [DOI] [PubMed] [Google Scholar]
- 33. Wong DE, Banyard DA, Santos PJF, Sayadi LR, Evans GRD, Widgerow AD. Adipose-derived stem cell extracellular vesicles: a systematic review. J Plast Reconstr Aesthet Surg. 2019;72(7):1207-1218. [DOI] [PubMed] [Google Scholar]
- 34. Rhode SC, Beier JP, Ruhl T. Adipose tissue stem cells in peripheral nerve regeneration—in vitro and in vivo. J Neurosci Res. 2021;99(2):545-560. [DOI] [PubMed] [Google Scholar]
- 35. Robinson JL, Feizi A, Uhlén M, Nielsen J. A systematic investigation of the malignant functions and diagnostic potential of the cancer secretome. Cell Rep. 2019;26(10):2622-2635.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kim J, Choi YS, Lim S, et al. Comparative analysis of the secretory proteome of human adipose stromal vascular fraction cells during adipogenesis. Proteomics. 2010;10(3):394-405. [DOI] [PubMed] [Google Scholar]
- 37. Lee MJ, Kim J, Kim MY, et al. Proteomic analysis of tumor necrosis factor-α-induced secretome of human adipose tissue-derived mesenchymal stem cells. J Proteome Res. 2010;9(4):1754-1762. [DOI] [PubMed] [Google Scholar]
- 38. Wang X, Lin Y. Tumor necrosis factor and cancer, buddies or foes? Acta Pharmacol Sin. 2008;29(11):1275-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zubkova ES, Beloglazova IB, Makarevich PI, et al. Regulation of adipose tissue stem cells angiogenic potential by tumor necrosis factor-alpha. J Cell Biochem. 2016;117(1):180-196. [DOI] [PubMed] [Google Scholar]
- 40. Kumari N, Dwarakanath BS, Das A, Bhatt AN. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumour Biol. 2016;37(9):11553-11572. [DOI] [PubMed] [Google Scholar]
- 41. Yuan A, Chen JJ, Yao PL, Yang PC. The role of interleukin-8 in cancer cells and microenvironment interaction. Front Biosci. 2005;10:853-865. [DOI] [PubMed] [Google Scholar]
- 42. Gialeli C, Theocharis AD, Karamanos NK. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J. 2011;278(1):16-27. [DOI] [PubMed] [Google Scholar]
- 43. Ritter A, Friemel A, Fornoff F, et al. Characterization of adipose-derived stem cells from subcutaneous and visceral adipose tissues and their function in breast cancer cells. Oncotarget. 2015;6(33):34475-34493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wei HJ, Zeng R, Lu JH, et al. Adipose-derived stem cells promote tumor initiation and accelerate tumor growth by interleukin-6 production. Oncotarget. 2015;6(10):7713-7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kengelbach-Weigand A, Tasbihi K, Strissel PL, et al. Plasticity of patient-matched normal mammary epithelial cells is dependent on autologous adipose-derived stem cells. Sci Rep. 2019;9(1):10722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Preisner F, Leimer U, Sandmann S, Zoernig I, Germann G, Koellensperger E. Impact of human adipose tissue-derived stem cells on malignant melanoma cells in an in vitro co-culture model. Stem Cell Rev Rep. 2018;14(1):125-140. [DOI] [PubMed] [Google Scholar]
- 47. Koellensperger E, Gramley F, Preisner F, Leimer U, Germann G, Dexheimer V. Alterations of gene expression and protein synthesis in co-cultured adipose tissue-derived stem cells and squamous cell-carcinoma cells: consequences for clinical applications. Stem Cell Res Ther. 2014;5(3):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang Y, Chu Y, Li K, et al. Exosomes secreted by adipose-derived mesenchymal stem cells foster metastasis and osteosarcoma proliferation by increasing COLGALT2 expression. Front Cell Dev Biol. 2020;8:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chu Y, Tang H, Guo Y, et al. Adipose-derived mesenchymal stem cells promote cell proliferation and invasion of epithelial ovarian cancer. Exp Cell Res. 2015;337(1):16-27. [DOI] [PubMed] [Google Scholar]
- 50. Naimo GD, Gelsomino L, Catalano S, Mauro L, Andò S. Interfering role of ERα on adiponectin action in breast cancer. Front Endocrinol (Lausanne). 2020;11:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sánchez-Jiménez F, Pérez-Pérez A, de la Cruz-Merino L, Sánchez-Margalet V. Obesity and breast cancer: role of leptin. Front Oncol. 2019;9:596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Delle Monache S, Calgani A, Sanità P, et al. Adipose-derived stem cells sustain prolonged angiogenesis through leptin secretion. Growth Factors. 2016;34(3-4):87-96. [DOI] [PubMed] [Google Scholar]
- 53. Strong AL, Ohlstein JF, Biagas BA, et al. Leptin produced by obese adipose stromal/stem cells enhances proliferation and metastasis of estrogen receptor positive breast cancers. Breast Cancer Res. 2015;17:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sabol RA, Villela VA, Denys A, et al. Obesity-altered adipose stem cells promote radiation resistance of estrogen receptor positive breast cancer through paracrine signaling. Int J Mol Sci. 2020;21(8):2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sabol RA, Beighley A, Giacomelli P, et al. Obesity-altered adipose stem cells promote ER+ breast cancer metastasis through estrogen independent pathways. Int J Mol Sci. 2019;20(6):1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sabol RA, Bowles AC, Côté A, et al. Leptin produced by obesity-altered adipose stem cells promotes metastasis but not tumorigenesis of triple-negative breast cancer in orthotopic xenograft and patient-derived xenograft models. Breast Cancer Res. 2019;21(1):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chow MT, Luster AD. Chemokines in cancer. Cancer Immunol Res. 2014;2(12):1125-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Weigand A, Boos AM, Tasbihi K, et al. Selective isolation and characterization of primary cells from normal breast and tumors reveal plasticity of adipose derived stem cells. Breast Cancer Res. 2016;18(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wang Y, Chu Y, Yue B, et al. Adipose-derived mesenchymal stem cells promote osteosarcoma proliferation and metastasis by activating the STAT3 pathway. Oncotarget. 2017;8(14):23803-23816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Takahara K, Ii M, Inamoto T, et al. microRNA-145 Mediates the inhibitory effect of adipose tissue-derived stromal cells on prostate cancer. Stem Cells Dev. 2016;25(17):1290-1298. [DOI] [PubMed] [Google Scholar]
- 61. Zeinali T, Mansoori B, Mohammadi A, Baradaran B. Regulatory mechanisms of miR-145 expression and the importance of its function in cancer metastasis. Biomed Pharmacother. 2019;109:195-207. [DOI] [PubMed] [Google Scholar]
- 62. Prieto González EA. Heterogeneity in adipose stem cells. Adv Exp Med Biol. 2019;1123:119-150. [DOI] [PubMed] [Google Scholar]
- 63. Li T, Zhou X, Wang J, et al. Adipose-derived mesenchymal stem cells and extracellular vesicles confer antitumor activity in preclinical treatment of breast cancer. Pharmacol Res. 2020;157:104843. [DOI] [PubMed] [Google Scholar]
- 64. Qu Y, Liu H, Lv X, et al. MicroRNA-16-5p overexpression suppresses proliferation and invasion as well as triggers apoptosis by targeting VEGFA expression in breast carcinoma. Oncotarget. 2017;8(42):72400-72410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. García-Contreras M, Vera-Donoso CD, Hernández-Andreu JM, García-Verdugo JM, Oltra E. Therapeutic potential of human adipose-derived stem cells (ADSCs) from cancer patients: a pilot study. PLoS One. 2014;9(11):e113288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ko SF, Yip HK, Zhen YY, et al. Adipose-derived mesenchymal stem cell exosomes suppress hepatocellular carcinoma growth in a rat model: apparent diffusion coefficient, natural killer T-cell responses, and histopathological features. Stem Cells Int. 2015;2015:853506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhao X, Wu D, Ma X, Wang J, Hou W, Zhang W. Exosomes as drug carriers for cancer therapy and challenges regarding exosome uptake. Biomed Pharmacother. 2020;128:110237. [DOI] [PubMed] [Google Scholar]
- 68. Lou G, Song X, Yang F, et al. Exosomes derived from miR-122-modified adipose tissue-derived MSCs increase chemosensitivity of hepatocellular carcinoma. J Hematol Oncol. 2015;8:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Jin MZ, Jin WL. The updated landscape of tumor microenvironment and drug repurposing. Signal Transduct Target Ther. 2020;5(1):166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bahrambeigi V, Ahmadi N, Salehi R, Javanmard SH. Genetically modified murine adipose-derived mesenchymal stem cells producing interleukin-2 favor B16F10 melanoma cell proliferation. Immunol Invest. 2015;44(3):216-236. [DOI] [PubMed] [Google Scholar]
- 71. Zhao C, Wu M, Zeng N, et al. Cancer-associated adipocytes: emerging supporters in breast cancer. J Exp Clin Cancer Res. 2020;39(1):156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Chait A, den Hartigh LJ. Adipose tissue distribution, inflammation and its metabolic consequences, including diabetes and cardiovascular disease. Front Cardiovasc Med. 2020;7:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sahai E, Astsaturov I, Cukierman E, et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat Rev Cancer. 2020;20(3):174-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kaur A, Ecker BL, Douglass SM, et al. Remodeling of the collagen matrix in aging skin promotes melanoma metastasis and affects immune cell motility. Cancer Discov. 2019;9(1):64-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Winkler J, Abisoye-Ogunniyan A, Metcalf KJ, Werb Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat Commun. 2020;11(1):5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Karnoub AE, Dash AB, Vo AP, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449(7162):557-563. [DOI] [PubMed] [Google Scholar]
- 77. Zhang Y, Daquinag AC, Amaya-Manzanares F, Sirin O, Tseng C, Kolonin MG. Stromal progenitor cells from endogenous adipose tissue contribute to pericytes and adipocytes that populate the tumor microenvironment. Cancer Res. 2012;72(20):5198-5208. [DOI] [PubMed] [Google Scholar]
- 78. Bochet L, Lehuédé C, Dauvillier S, et al. Adipocyte-derived fibroblasts promote tumor progression and contribute to the desmoplastic reaction in breast cancer. Cancer Res. 2013;73(18):5657-5668. [DOI] [PubMed] [Google Scholar]
- 79. Miyazaki Y, Oda T, Mori N, Kida YS. Adipose-derived mesenchymal stem cells differentiate into pancreatic cancer-associated fibroblasts in vitro. FEBS Open Bio. 2020;10(11):2268-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Miyazaki Y, Oda T, Inagaki Y, et al. Adipose-derived mesenchymal stem cells differentiate into heterogeneous cancer-associated fibroblasts in a stroma-rich xenograft model. Sci Rep. 2021;11(1):4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Przybyt E, van Luyn MJ, Harmsen MC. Extracellular matrix components of adipose derived stromal cells promote alignment, organization, and maturation of cardiomyocytes in vitro. J Biomed Mater Res A. 2015;103(5):1840-1848. [DOI] [PubMed] [Google Scholar]
- 82. Seo BR, Bhardwaj P, Choi S, et al. Obesity-dependent changes in interstitial ECM mechanics promote breast tumorigenesis. Sci Transl Med. 2015;7(301):301ra130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Madu CO, Wang S, Madu CO, Lu Y. Angiogenesis in breast cancer progression, diagnosis, and treatment. J Cancer. 2020;11(15):4474-4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Castro-Oropeza R, Vazquez-Santillan K, Díaz-Gastelum C, et al. Adipose-derived mesenchymal stem cells promote the malignant phenotype of cervical cancer. Sci Rep. 2020;10(1):14205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Zhang Y, Daquinag A, Traktuev DO, et al. White adipose tissue cells are recruited by experimental tumors and promote cancer progression in mouse models. Cancer Res. 2009;69(12):5259-5266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lamfers M, Idema S, van Milligen F, et al. Homing properties of adipose-derived stem cells to intracerebral glioma and the effects of adenovirus infection. Cancer Lett. 2009;274(1):78-87. [DOI] [PubMed] [Google Scholar]
- 87. Gehmert S, Gehmert S, Prantl L, Vykoukal J, Alt E, Song YH. Breast cancer cells attract the migration of adipose tissue-derived stem cells via the PDGF-BB/PDGFR-beta signaling pathway. Biochem Biophys Res Commun. 2010;398(3):601-605. [DOI] [PubMed] [Google Scholar]
- 88. Kidd S, Spaeth E, Watson K, et al. Origins of the tumor microenvironment: quantitative assessment of adipose-derived and bone marrow-derived stroma. PLoS One. 2012;7(2):e30563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Bellows CF, Zhang Y, Chen J, Frazier ML, Kolonin MG. Circulation of progenitor cells in obese and lean colorectal cancer patients. Cancer Epidemiol Biomarkers Prev. 2011;20(11):2461-2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Loebinger MR, Eddaoudi A, Davies D, Janes SM. Mesenchymal stem cell delivery of TRAIL can eliminate metastatic cancer. Cancer Res. 2009;69(10):4134-4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Zhang Y, Weinberg RA. Epithelial-to-mesenchymal transition in cancer: complexity and opportunities. Front Med. 2018;12(4):361-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Wu S, Wang Y, Yuan Z, et al. Human adipose-derived mesenchymal stem cells promote breast cancer MCF7 cell epithelial-mesenchymal transition by cross interacting with the TGF-β/Smad and PI3K/AKT signaling pathways. Mol Med Rep. 2019;19(1):177-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Park YM, Yoo SH, Kim SH. Adipose-derived stem cells induced EMT-like changes in H358 lung cancer cells. Anticancer Res. 2013;33(10):4421-4430. [PubMed] [Google Scholar]
- 94. Iser IC, Ceschini SM, Onzi GR, Bertoni AP, Lenz G, Wink MR. Conditioned medium from adipose-derived stem cells (ADSCs) promotes epithelial-to-mesenchymal-like transition (EMT-Like) in glioma cells in vitro. Mol Neurobiol. 2016;53(10):7184-7199. [DOI] [PubMed] [Google Scholar]
- 95. Bahmad HF, Cheaito K, Chalhoub RM, et al. Sphere-formation assay: three-dimensional in vitro culturing of prostate cancer stem/progenitor sphere-forming cells. Front Oncol. 2018;8:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ceccarelli S, Pontecorvi P, Anastasiadou E, Napoli C, Marchese C. Immunomodulatory effect of adipose-derived stem cells: the cutting edge of clinical application. Front Cell Dev Biol. 2020;8:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Muz B, de la Puente P, Azab F, Azab AK. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia (Auckl). 2015;3:83-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. de la Cruz-Merino L, Chiesa M, Caballero R, et al. Breast cancer immunology and immunotherapy: current status and future perspectives. Int Rev Cell Mol Biol. 2017;331:1-53. [DOI] [PubMed] [Google Scholar]
- 99. Razmkhah M, Jaberipour M, Erfani N, Habibagahi M, Talei AR, Ghaderi A. Adipose derived stem cells (ASCs) isolated from breast cancer tissue express IL-4, IL-10 and TGF-β1 and upregulate expression of regulatory molecules on T cells: do they protect breast cancer cells from the immune response? Cell Immunol. 2011;266(2):116-122. [DOI] [PubMed] [Google Scholar]
- 100. Fakhimi M, Talei AR, Ghaderi A, Habibagahi M, Razmkhah M. Helios, CD73 and CD39 induction in regulatory T cells exposed to adipose derived mesenchymal stem cells. Cell J. 2020;22(2):236-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Viguier M, Lemaître F, Verola O, et al. Foxp3 expressing CD4+CD25high regulatory T cells are overrepresented in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells. J Immunol. 2004;173(2):1444-1453. [DOI] [PubMed] [Google Scholar]
- 102. Sineh Sepehr K, Razavi A, Hassan ZM, et al. Comparative immunomodulatory properties of mesenchymal stem cells derived from human breast tumor and normal breast adipose tissue. Cancer Immunol Immunother. 2020;69(9):1841-1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Razmkhah M, Abedi N, Hosseini A, Imani MT, Talei AR, Ghaderi A. Induction of T regulatory subsets from naïve CD4+ T cells after exposure to breast cancer adipose derived stem cells. Iran J Immunol. 2015;12(1):1-15. [PubMed] [Google Scholar]
- 104. Bahrami B, Hosseini A, Talei AR, Ghaderi A, Razmkhah M. Adipose derived stem cells exert immunomodulatory effects on natural killer cells in breast cancer. Cell J. 2017;19(1):137-145. [PMC free article] [PubMed] [Google Scholar]
- 105. Mehdipour F, Razmkhah M, Rezaeifard S, et al. Mesenchymal stem cells induced anti-inflammatory features in B cells from breast tumor draining lymph nodes. Cell Biol Int. 2018;42(12):1658-1669. [DOI] [PubMed] [Google Scholar]
- 106. Razmkhah M, Mansourabadi Z, Mohtasebi MS, Talei AR, Ghaderi A. Cancer and normal adipose-derived mesenchymal stem cells (ASCs): do they have differential effects on tumor and immune cells? Cell Biol Int. 2018;42(3):334-343. [DOI] [PubMed] [Google Scholar]
- 107. Benaiges E, Ceperuelo-Mallafré V, Madeira A, et al. Survivin drives tumor-associated macrophage reprogramming: a novel mechanism with potential impact for obesity. Cell Oncol (Dordr). 2021;44(4):777-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Al-Ghadban S, Bunnell BA. Adipose tissue-derived stem cells: immunomodulatory effects and therapeutic potential. Physiology (Bethesda). 2020;35(2):125-133. [DOI] [PubMed] [Google Scholar]
- 109. Kilinc MO, Santidrian A, Minev I, et al. The ratio of ADSCs to HSC-progenitors in adipose tissue derived SVF may provide the key to predict the outcome of stem-cell therapy. Clin Transl Med. 2018;7(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Blaber SP, Webster RA, Hill CJ, et al. Analysis of in vitro secretion profiles from adipose-derived cell populations. J Transl Med. 2012;10:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Lee JS, Eo P, Kim MC, et al. Effects of stromal vascular fraction on breast cancer growth and fat engraftment in NOD/SCID mice. Aesthetic Plast Surg. 2019;43(2):498-513. [DOI] [PubMed] [Google Scholar]
- 112. Mazur S, Zołocińska A, Siennicka K, Janik-Kosacka K, Chrapusta A, Pojda Z. Safety of adipose-derived cell (stromal vascular fraction—SVF) augmentation for surgical breast reconstruction in cancer patients. Adv Clin Exp Med. 2018;27(8):1085-1090. [DOI] [PubMed] [Google Scholar]
- 113. Calabrese C, Kothari A, Badylak S, et al. Oncological safety of stromal vascular fraction enriched fat grafting in two-stage breast reconstruction after nipple sparing mastectomy: long-term results of a prospective study. Eur Rev Med Pharmacol Sci. 2018;22(15):4768-4777. [DOI] [PubMed] [Google Scholar]
- 114. Hübner J, Katalinic A, Waldmann A, Kraywinkel K. Long-term incidence and mortality trends for breast cancer in Germany. Geburtshilfe Frauenheilkd. 2020;80(6):611-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Koellensperger E, Bonnert LC, Zoernig I, et al. The impact of human adipose tissue-derived stem cells on breast cancer cells: implications for cell-assisted lipotransfers in breast reconstruction. Stem Cell Res Ther. 2017;8(1):121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Rowan BG, Gimble JM, Sheng M, et al. Human adipose tissue-derived stromal/stem cells promote migration and early metastasis of triple negative breast cancer xenografts. PLoS One. 2014;9(2):e89595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Eterno V, Zambelli A, Pavesi L, et al. Adipose-derived mesenchymal stem cells (ASCs) may favour breast cancer recurrence via HGF/c-Met signaling. Oncotarget. 2014;5(3):613-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Xu H, Li W, Luo S, Yuan J, Hao L. Adipose derived stem cells promote tumor metastasis in breast cancer cells by stem cell factor inhibition of miR20b. Cell Signal. 2019;62:109350. [DOI] [PubMed] [Google Scholar]
- 119. Fajka-Boja R, Szebeni GJ, Hunyadi-Gulyás É, Puskás LG, Katona RL. Polyploid adipose stem cells shift the balance of IGF1/IGFBP2 to promote the growth of breast cancer. Front Oncol. 2020;10:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Lin R, Wang S, Zhao RC. Exosomes from human adipose-derived mesenchymal stem cells promote migration through Wnt signaling pathway in a breast cancer cell model. Mol Cell Biochem. 2013;383(1-2):13-20. [DOI] [PubMed] [Google Scholar]
- 121. Kucerova L, Skolekova S, Matuskova M, Bohac M, Kozovska Z. Altered features and increased chemosensitivity of human breast cancer cells mediated by adipose tissue-derived mesenchymal stromal cells. BMC Cancer. 2013;13:535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Petit JY, Maisonneuve P, Rotmensz N, Bertolini F, Rietjens M. Fat grafting after invasive breast cancer: a matched case-control study. Plast Reconstr Surg. 2017;139(6):1292-1296. [DOI] [PubMed] [Google Scholar]
- 123. Cohen O, Lam G, Karp N, Choi M. Determining the oncologic safety of autologous fat grafting as a reconstructive modality: an institutional review of breast cancer recurrence rates and surgical outcomes. Plast Reconstr Surg. 2017;140(3):382e-392e. [DOI] [PubMed] [Google Scholar]
- 124. Kaoutzanis C, Xin M, Ballard TN, et al. Autologous fat grafting after breast reconstruction in postmastectomy patients: complications, biopsy rates, and locoregional cancer recurrence rates. Ann Plast Surg. 2016;76(3):270-275. [DOI] [PubMed] [Google Scholar]
- 125. Myckatyn TM, Wagner IJ, Mehrara BJ, et al. Cancer risk after fat transfer: a multicenter case-cohort study. Plast Reconstr Surg. 2017;139(1):11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Pérez-Cano R, Vranckx JJ, Lasso JM, et al. Prospective trial of adipose-derived regenerative cell (ADRC)-enriched fat grafting for partial mastectomy defects: the RESTORE-2 trial. Eur J Surg Oncol. 2012;38(5):382-389. [DOI] [PubMed] [Google Scholar]
- 127. Petit JY, Lohsiriwat V, Clough KB, et al. The oncologic outcome and immediate surgical complications of lipofilling in breast cancer patients: a multicenter study—Milan-Paris-Lyon experience of 646 lipofilling procedures. Plast Reconstr Surg. 2011;128(2):341-346. [DOI] [PubMed] [Google Scholar]
- 128. Hanson SE, Kapur SK, Garvey PB, et al. Oncologic safety and surveillance of autologous fat grafting following breast conservation therapy. Plast Reconstr Surg. 2020;146(2):215-225. [DOI] [PubMed] [Google Scholar]
- 129. Cason RW, Shammas RL, Broadwater G, et al. The influence of fat grafting on breast imaging after postmastectomy reconstruction: a matched cohort analysis. Plast Reconstr Surg. 2020;146(6):1227-1236. [DOI] [PubMed] [Google Scholar]
- 130. Perrot P, Rousseau J, Bouffaut AL, et al. Safety concern between autologous fat graft, mesenchymal stem cell and osteosarcoma recurrence. PLoS One. 2010;5(6):e10999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Rodriguez C, Freedland SJ, Deka A, et al. Body mass index, weight change, and risk of prostate cancer in the Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiol Biomarkers Prev. 2007;16(1):63-69. [DOI] [PubMed] [Google Scholar]
- 132. Clarke MA, Fetterman B, Cheung LC, et al. Epidemiologic evidence that excess body weight increases risk of cervical cancer by decreased detection of precancer. J Clin Oncol. 2018;36(12):1184-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Foong KW, Bolton H. Obesity and ovarian cancer risk: a systematic review. Post Reprod Health. 2017;23(4):183-198. [DOI] [PubMed] [Google Scholar]
- 134. Freedland SJ, Bañez LL, Sun LL, Fitzsimons NJ, Moul JW. Obese men have higher-grade and larger tumors: an analysis of the duke prostate center database. Prostate Cancer Prostatic Dis. 2009;12(3):259-263. [DOI] [PubMed] [Google Scholar]
- 135. Zhai Y, Wu W, Xi X, Yu R. Adipose-derived stem cells promote proliferation and invasion in cervical cancer by targeting the HGF/c-MET pathway. Cancer Manag Res. 2020;12:11823-11832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Chu Y, You M, Zhang J, et al. Adipose-derived mesenchymal stem cells enhance ovarian cancer growth and metastasis by increasing thymosin beta 4X-linked expression. Stem Cells Int. 2019;2019:9037197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Chu Y, Zhu C, Wang Q, et al. Adipose-derived mesenchymal stem cells induced PAX8 promotes ovarian cancer cell growth by stabilizing TAZ protein. J Cell Mol Med. 2021;25(9):4434-4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Charles-de-Sá L, Gontijo-de-Amorim N, Sbarbati A, et al. Photoaging skin therapy with PRP and ADSC: a comparative study. Stem Cells Int. 2020;2020:2032359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Kim WS, Park BS, Sung JH. The wound-healing and antioxidant effects of adipose-derived stem cells. Expert Opin Biol Ther. 2009;9(7):879-887. [DOI] [PubMed] [Google Scholar]
- 140. Park HS, Son HY, Choi MH, et al. Adipose-derived stem cells attenuate atopic dermatitis-like skin lesions in NC/Nga mice. Exp Dermatol. 2019;28(3):300-307. [DOI] [PubMed] [Google Scholar]
- 141. Ahn JO, Coh YR, Lee HW, Shin IS, Kang SK, Youn HY. Human adipose tissue-derived mesenchymal stem cells inhibit melanoma growth in vitro and in vivo. Anticancer Res. 2015;35(1):159-168. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analyzed in support of this research.