Abstract
The respiratory syncytial virus (RSV) fusion (F) protein undergoes two furin-cleavage events to become fusion competent, resulting in the release of a twenty-seven amino acid peptide (p27). Recent studies indicate that the p27 region of the F protein was an immunodominant antigen in young children. In this study, we evaluated the kinetics of the serum antibody response to the p27 peptide following natural RSV reinfection in adults. Nineteen healthy adults under sixty-five years of age were enrolled during the 2018–2019 RSV season in Houston, TX. Blood was collected at three study visits and RSV infection status was defined by changes in neutralizing antibody resulting in three groups: uninfected (n=12), acutely infected (n=4), and recently infected (n=3). Serum IgG and IgA antibodies against RSV/A and RSV/B p27 peptides were measured by enzyme-linked immunosorbent assays, and serum p27-like antibodies were detected by a p27 competitive antibody assay. Anti-p27 antibodies were detected in all subjects at each study visit. The measured IgG and IgA anti-p27 antibody levels followed the same pattern as other RSV site-specific and neutralizing antibody responses described for this cohort previously: the uninfected group had stable responses for the duration of the study period, the acutely infected group had a significant increase following RSV infection, and the recently infected group had a decrease in anti-p27 antibody during the study period. These results indicate that antibodies to the p27 region of the F protein are generated following natural RSV reinfection and suggest that some of the F protein is potentially in a partially cleaved state on the surface of virions, expanding on the previous assumption that all of p27 is post-translationally released and not present on mature F. Additionally, antibody responses were significantly lower (1.4–1.5-fold) toward RSV/B than to RSV/A p27 at each study visit, despite being an RSV/B dominant outbreak. Understanding the mechanism for the differences in the magnitude of the RSV/A and RSV/B p27 antibody response may enhance our understanding of the intracellular processing of the F protein.
Keywords: respiratory syncytial virus, p27 peptide, furin cleavage site, fusion protein, pneumovirus
1. Introduction
Human respiratory syncytial virus (RSV) is one of the leading causes of acute lower respiratory infections (ALRI) worldwide, imposing a significant impact on global public health [1–3]. A ubiquitous pathogen, RSV infects all children at least once by their third birthday, with more than half acquiring two or more RSV infections within that period [4]. RSV also poses a substantial health threat to the elderly and high-risk adults, with a disease burden similar to influenza [5]. Despite over 60 years of pursuit, no licensed vaccines are yet available. Tremendous efforts in the last 5–10 years, however, have led to a surge in vaccine candidates in preclinical and clinical development [6].
RSV is an enveloped, negative sense single-stranded RNA virus that belongs to the Orthopneumovirus genus of the Pneumoviridae family in the order Mononegavirales. It encodes 11 proteins, three of which are expressed on the viral envelope: the attachment (G), the small hydrophobic (SH), and the fusion (F) proteins [7]. The F protein, which mediates fusion between the viral and host cell membranes, is the primary focus of the neutralizing antibody response [8, 9]. The RSV F protein is conserved across the two RSV subtypes, RSV/A and RSV/B [10, 11]. For these reasons, the majority of vaccine candidates and antiviral drugs in development target the F protein [6].
Uniquely among the pneumoviruses, the RSV F protein undergoes two cleavage events by furin-like proteases at highly conserved polybasic cleavage sites (site I, RARR109, and site II, KKRKRR136) [12]. The F protein is initially synthesized as a 70 kDa inactive precursor (F0). F0 is processed in the trans-Golgi during intracellular maturation before becoming expressed on the surface of infected cells. Like many viral envelope proteins, the RSV F protein is co- and post- translationally modified with 5 or 6 N-linked glycans during transport through the secretory pathway, depending on the viral strain [11, 13]. At least two of the potential N-linked glycans are found within the p27 region. Cleavage at both furin sites releases a twenty-seven amino acid peptide (p27, aa 109–136) and results in two subunits, F1 and F2 (50 kDa and 20 kDa, respectively), held together by disulfide bonds. Proteolytic processing at both cleavage sites is necessary for the F protein to become fusion competent [14].
Previous studies have found that, as with other paramyxoviruses, RSV fuses its membrane directly with that of target cells [15]. This model is supported by furin-like enzymes present in the trans-Golgi that can cleave both sites and results in expression of the F protein on the surface of the plasma membrane [16, 17]. In this model, the prefusion F protein trimerizes to generate mature, fully active F protein [18] and is supported by the fact that RSV entry is pH-independent [19]. The first model assumes that F protein on the membrane of infected cells or surface of virions lacks the p27 peptide and that free p27 is generated in the trans-Golgi during intracellular F maturation.
Recent evidence, however, indicates that RSV entry may also occur by a two-step process, whereby RSV is first endocytosed and fusion occurs within endosomes [20, 21]. Krzyzaniak et al., found that this uptake is an actin-dependent process with the hallmarks of macropinocytosis [22]. Upon uptake within macropinosomes, furin-like proteases remove a small peptide, consistent with the size of p27, resulting in fusion-competent F protein. In the second model, then, the F protein undergoes a single cleavage within the trans-Golgi, is expressed on the surface of cells in a partially activated state, and is followed by viral assembly and release of the virion. Viral entry occurs after uptake by macropinosomes, where a second cleavage event leads to fusion competent F protein, leading to subsequent fusion of virus and host membranes and viral entry. In this second model, p27 is bound to F1 at the time of infection and free p27 is generated intracellularly after virus entry. In agreement with this model, a recent study found that the F0 form of the F protein, which contains p27, is expressed on the surface of a large proportion of RSV-infected lung epithelial cells in vivo [23].
In a recent study, the antibody repertoire following primary RSV infection through adulthood was analyzed using a whole genome-fragment phage display library. Unexpectedly, sera from children < 2 years of age strongly bound aa 101–121 of the F protein, corresponding with the p27 peptide, whereas sera in adults bound much less tightly to this region, indicating that p27 is an age-dependent immunodominant epitope [24]. Follow-up studies on p27 antibody responses in hematopoietic stem cell transplant patients found that specifically the mucosal p27 antibody response may help to control RSV infection [25, 26]. Additionally, immunization of mice with a DNA plasmid expressing a mutated F protein (N116Q) lacking the N glycosylation sequon within the p27 domain resulted in increased neutralizing antibody responses and enhanced protection from subsequent viral infection. These results suggest that this glycan masks a protective epitope on p27 [27]. Additionally, immunization with a non-glycosylated p27 peptide provided protection against RSV infection in murine challenge studies [23]. Further studies are needed to better understand the role of p27 in RSV infection and protection. Here we assessed antibody responses to the p27 region of RSV/A and RSV/B subtypes in healthy adults that were either uninfected or had been naturally reinfected over the course of a single RSV season to address this question.
2. Materials and Methods
2.1. Study Design
Nineteen healthy adults enrolled and completed a longitudinal prospective study during the 2018–2019 RSV season in Houston, Texas as described previously [28]. Blood samples were collected in SST tubes at three time points (visits 1, 2, and 3), which occurred in November 2018, January 2019, and May 2019, respectively. RSV infection status was determined by four-fold or greater changes in neutralizing antibody titers over time using qualified microneutralization assays [29]. Volunteers with less than a four-fold change in neutralizing activity over the course of the season were defined as uninfected, those with four-fold or greater increases between two consecutive study visits were defined as having an acute RSV infection, and those with a four-fold or greater decrease in neutralizing antibody titer at their second visit were defined as having a recent infection prior to enrollment, indicating we missed their baseline titer prior to their RSV infection [28].
2.2. Ethics Statement
The institutional review board at Baylor College of Medicine approved the study protocol and written informed consent was obtained from all enrolled participants.
2.3. Biotinylated RSV p27 Peptide
The consensus sequence of RSV/A and RSV/B p27 peptides ([NH2]ELPRFMNYTLNNTKNTNVTLSKKRKRR[COOH] and [NH2]EAPQYMNYTINTTKNLNVSISKKRKRR[COOH], respectively) were obtained from published data from our group [10]. The accuracy of the consensus sequences were confirmed by NCBI BLAST searches with 100% query cover and 100% identity to each respective subgroup. The RSV/A and RSV/B p27 peptides were chemically synthesized and biotinylated by Thermo Fisher Scientific. The p27 peptides had > 97% purity by analytical High Performance Liquid Chromatography and the amino acid components were verified by mass spectrometry. To allow for binding of antibody to the p27 peptide in ELISA with the least possible steric hindrance, an extra lysine, an aminohexanoic acid (Ahx) spacer (a more cost effective and more hydrophobic spacer compared to polyethylene glycol), and biotin were sequentially added to the C-terminus of the peptide (ThermoFisher Scientific, Waltham, MA). The final biotinylated RSV/A p27 peptide used in this study was, thus, [NH2]ELPRFMNYTLNNTKNTNVTLSKKRKRR-Lys[COOH](Ahx-biotin). The final biotinylated RSV/B p27 peptide was [NH2]EAPQYMNYTINTTKNLNVSISKKRKRR- Lys[COOH](Ahx-biotin).
2.4. Biotinylated RSV p27 Monoclonal Antibody
The mouse anti-human p27 monoclonal antibody, mAb RSV7.10, was kindly provided by Dr. Gale Smith (Novavax, Gaithersburg, MD). The Pierce Antibody Biotinylation Kit for IP (Cat. # 90407, ThermoFisher Scientific, Waltham, MA) was used to biotinylate the mAb according to the manufacturer’s instructions with some modifications. Briefly, 100 μg of p27 mAb (100 μL of 1 mg/mL) was buffer exchanged in 1X PBS for compatibility with the biotinylation reaction. 100 μL of 1X PBS was then added directly to the kit-supplied NHS-PEG4-Biotin to a concentration of 8.5 mM. 3.14 μL of the biotin solution was then added to 96.84 μL of purified p27 mAb in a 1.5 mL microcentrifuge tube for a total of 100 μL per reaction. The mixture was gently rotated at 25°C in the dark for 1 h. Finally, the buffer was again exchanged to remove unbound biotin. The biotinylated p27 mAb stocks were stored in one-use aliquots at −80°C until use.
2.5. p27 Enzyme-Linked Immunosorbent Assays
RSV/A and RSV/B p27-specific antibodies (serum IgA and IgG anti-p27 peptide) were quantified by ELISA as described previously [26]. Briefly, streptavidin-labeled 96-well plates (Cat. # 15124, Thermo Scientific) were coated with either RSV/A or RSV/B consensus biotinylated p27 peptide. Serum was added in duplicate followed by 2-fold serial dilutions (1:20–1:2,560). Anti-p27 mAb RSV7.10 was added and serially diluted 2-fold from 10 ng/mL to 0.156 ng/mL to generate a standard curve. Each concentration was done in duplicate. HRP-conjugated secondary antibody (anti-mouse IgG, Bio-Rad Laboratories, Inc. Richmond, CA), was added to the p27 mAb standard curve samples and anti-human IgG (Bio-Rad Laboratories, Inc. Richmond, CA) or anti-human IgA (ThermoFisher Scientific, Waltham, MA), was added to the study samples. A four-parameter logistic regression model was used to calculate the p27 antibody concentrations, in ng/mL, based on the dynamic range of the standard curve. The lower limit of detection (LLoD) was 0.2 ng/mL, and a negative sample was assigned a value of 0.1 ng/mL, as described previously [26,30].
2.6. p27 Competitive Antibody Assay
The p27 competitive assay measures antibodies that inhibit RSV7.10 from binding to p27 otherwise known as p27-like antibodies (P27LA) as described previously [26]. A standard curve of p27 mAb was generated on each plate. Serial 2-fold dilutions of test sera (1:10–1:1,280), in duplicate, were added to the coated plates, followed by the addition of biotinylated p27 mAb to the entire plate. Wells containing biotinylated p27 mAb without serum samples served as positive controls representing maximum binding. Wells not coated with F protein served as negative controls. A four-parameter logistic regression model was used to calculate the P27LA concentrations (in ng/mL) based on the dynamic range of the standard curve by interpolating the concentration of the standards that corresponds to the absorbance value at which the test sample resulted in 50% inhibition. The LLoD was 48.8 ng/mL for the P27CA assay. Samples with concentration below the LLoD were assigned a half value of the LLoD as described previously [26,30].
2.7. RSV-Specific Microneutralization Assay
Heat-inactivated serum samples were analyzed for neutralizing antibodies against prototypic (RSV/A/Tracy and RSV/B/18537) and contemporaneous (RSV/A/Ontario and RSV/B/Buenos Aires) strains in HEp-2 cells using qualified microneutralization assays as described previously [29]. Neutralizing antibody titers were defined as the highest dilution at which there was a ≥ 50% reduction in viral cytopathic effect. Any serum sample resulting in a titer less than the lower limit of detection (LLoD) (2.5 log2) was assigned a value of 2 log2 as described in [28,30].
2.8. Palivizumab competitive assay
A competitive antibody assay was used to measure serum concentrations of palivizumab-competing antibody (site II) as described in detail previously [28,31,32]. Palivizumab was sourced from MedImmune, LLC, Gaithersburg, MD, USA. A four-parameter logistic regression model was used to calculate the competitive antibody concentrations (μg/mL) that resulted in ≥50% inhibition. The LLoD was 1.0 μg/mL II, and samples with a concentration below the LLoD were assigned a value of 0.5 μg/mL, respectively, as described in [28,30].
2.8. Statistical Analyses
A repeated-measures mixed model was performed to test for differences in the means of log2 transformed geometric mean concentration (GMC) for IgG and IgA anti-p27 binding antibody concentrations (ng/mL), and p27 competitive antibody concentrations (ng/mL) among the three infection status groups and three study visits. Pairwise comparisons were performed of the post-estimated mean log-transformed antibody concentrations among the three groups. Statistical significance (with greater than 95% confidence) was indicated for p values ≤ 0.05. No correction was made for multiple comparisons. Pearson’s correlation coefficients were calculated between IgG and IgA anti p27 antibodies, p27 competitive antibody concentrations, and each of the RSV/A and RSV/B neutralizing antibody titers, as well as palivizumab-like antibody (PLA) concentrations, which measures antibody that can compete with the clinically relevant immunoprophylactic palivizumab. Statistical analyses were performed using Stata 14 (Stata Corp, College Station, Texas).
3. Results
3.1. Demographics
Healthy adults under the age of 65 with no underlying conditions were enrolled during the 2018–2019 RSV/B dominant season, as described previously [28]. There were three groups of RSV infection status, which were defined by changes in neutralizing antibody titer: uninfected (n=12), acutely infected (n=4), and recently infected (n=3). Ages ranged from 23–59, with no discernable difference we could detect among age, sex, or ethnicity across infection status [28].
3.2. IgG anti-p27 antibodies in healthy adults
All 19 enrolled healthy adults had detectable levels of serum IgG anti-p27 antibodies (Table 1) at all three study visits. The measured IgG anti-p27 antibody levels followed the same pattern as other RSV site-specific and neutralizing antibody responses described for this cohort previously [28]: the uninfected group had stable responses for the duration of the study period (185 days), the acutely infected group had a significant increase following RSV infection, and the recently infected group had a decrease in IgG anti-p27 antibody titers over the course of the RSV season.
Table 1.
IgG anti-p27 antibody concentrations in sera from healthy adults over an RSV season
| Serum IgG anti-p27 a | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| GMC | Fold Changeb | |||||||||
| Infection Status | Visit 1 | Visit 2 | Visit 3 | Visit 1–2 | p Value | Visit 1–3 | p Value | Visit 2–3 | p Value | |
| Uninfected | RSV/A | 460.4 (345.6–613.2) | 461.7 (357.7–595.8) | 462.1 (357.6–597.2) | 1.0 | 0.819 | 1.0 | 0.888 | 1.0 | 0.930 |
| RSV/B | 368.4 (304.0–446.4) | 347.7 (279.1–433.2) | 329.3 (258.1–420.3) | −1.1 | 0.388 | −1.1 | 0.121 | −1.1 | 0.478 | |
| Acutely Infected | RSV/A | 392.8 (290.0–532.1) | 571.7 (345.9–944.9) | 740.5 (399.0–1374.6) | 1.5 | 0.002 | 1.9 | 0.000 | 1.3 | 0.003 |
| RSV/B | 237.0 (175.0–321.1) | 378.1 (294.0–486.4) | 517.9 (407.4–658.5) | 1.6 | 0.000 | 2.2 | 0.000 | 1.4 | 0.001 | |
| Recent Infection | RSV/A | 648.9 (395.8–1063.9) | 517.8 (310.5–863.6) | 526.4 (325.0–852.8) | −1.3 | 0.078 | −1.2 | 0.085 | 1.0 | 0.964 |
| RSV/B | 439.8 (311.5–621.0) | 275.8 (485.1–156.8) | 279.8 (440.1–177.9) | −1.6 | 0.000 | −1.6 | 0.000 | 1.0 | 0.935 | |
Geometric mean concentration (ng/mL) for serum (95% confidence interval) IgG anti-p27 antibodies in healthy adults with or without a serologically defined RSV infection.
Fold-change in serum anti-p27 antibody titer between study visits is shown. Pairwise comparisons in post-estimated GMNAT between visits within each group was tested and considered significant at p <0.05.
The GMC of IgG anti-RSV/A p27 antibody levels of uninfected adults remained stable during the RSV season (460.4–462.1 ng/mL). Following infection, the GMC of IgG anti-RSV/A p27 antibody of acutely infected adults rose significantly (Visit 2: 571.7 ng/mL and Visit 3: 740.5 ng/mL) and were comparable to levels measured in recently infected adults at Visit 1 (648.9 ng/mL). In the acutely infected group, IgG anti-RSV/A p27 responses continued to rise significantly between the second and third study visit. In recently infected adults, the GMC of IgG anti-RSV/A p27 antibody titers decreased over the duration of the study period, though this change was not statistically significant.
Because the sequence of p27 varies between the RSV/A and RSV/B subtypes, we measured IgG antibody responses to the consensus RSV/B p27 peptide to compare the antibody responses to each subtype. IgG anti-RSV/B p27 responses largely follow the same pattern observed for IgG anti-RSV/A p27 responses (Table 1); GMC of IgG anti-RSV/B p27 antibody titers remained stable in the uninfected group, demonstrated a GMC increase with significant rises between each of the study visits in the acutely infected group, and resulted in a significant decrease between the first and second study visits that remained stable between the second and third study visits in the recently infected group.
To determine whether antibody responses to each subtype differed, we compared IgG anti-RSV/A p27 and IgG anti-RSV/B p27 GMC at each timepoint by infection status using a one-way ANOVA. IgG anti-RSV/A p27 antibody levels were significantly higher than IgG anti-RSV/B p27 antibody responses (Visit 1: p = 0.052, Visit 2: p = 0.027, Visit 3: p = 0.009) by a magnitude of 1.4–1.5 fold, respectively.
3.3. IgA anti-p27 antibodies in healthy adults
All individuals enrolled in the study also had detectable levels of serum IgA anti-p27 antibody during all three study visits (Table 2). The GMC of serum IgA anti-RSV/A p27 responses did not change in the uninfected group during the RSV season (39.5–43.2 ng/mL). There was not a significant change in either the IgA anti-RSV/A p27 in the acutely or recently infected groups between the first and second study visits. Between the first and third study visits, however, there was a significant rise in the acutely infected group and significant decrease in the recently infected group. The GMC of IgA anti-RSV/B p27 antibody significantly increased between all study visits in the acutely infected group. In the recently infected group, there was no statistically significant change in GMC of IgA anti RSV/B p27 antibody over the study period. Unlike with IgG anti-p27 responses between RSV subtypes, however, there was no significant difference in the GMC between IgA anti-RSV/A p27 and IgA anti-RSV/B p27 (Visit 1: p =0.979, fold difference = 1.0; Visit 2: p =0.886, fold difference = 1.1; Visit 3: p = 0.996, fold difference = 1.1).
Table 2.
IgA anti-p27 antibody concentrations in sera from healthy adults over an RSV season
| Serum IgA anti-p27 a | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| GMC | Fold Changeb | |||||||||
| Infection Status | Visit 1 | Visit 2 | Visit 3 | Visit 1–2 | p Value | Visit 1–3 | p Value | Visit 2–3 | p Value | |
| Uninfected | RSV/A | 42.6 (29.9–60.5) | 37.7 (30.5–46.7) | 41.2 (31.8–53.5) | −1.1 | 0.085 | 1.1 | 0.384 | −1.0 | 0.379 |
| RSV/B | 43.2 (33.6–55.5) | 42.0 (35.2–50.1) | 39.5 (31.6–49.3) | −1.0 | 0.475 | −1.1 | 0.290 | −1.1 | 0.726 | |
| Acutely Infected | RSV/A | 39.2 (22.8–67.6) | 51.9 (38.1–70.6) | 56.3 (33.0–96.1) | 1.3 | 0.073 | 1.4 | 0.001 | 1.1 | 0.092 |
| RSV/B | 48.3 (22.5–103.8) | 67.8 (48.4–95.1) | 73.5 (44.2–122.1) | 1.4 | 0.003 | 1.5 | 0.000 | 1.1 | 0.004 | |
| Recent Infection | RSV/A | 73.5 (56.2–96.2) | 51.2 (34.4–76.1) | 40.9 (21.0–79.5) | −1.4 | 0.099 | −1.8 | 0.029 | −1.3 | 0.561 |
| RSV/B | 54.4 (23.4–84.2) | 49.9 (42.0–59.1) | 49.3 (38.8–62.7) | −1.1 | 0.959 | −1.1 | 0.973 | −1.0 | 0.986 | |
Geometric mean concentration (ng/mL) for serum (95% confidence interval) IgG anti-p27 antibodies in healthy adults with or without a serologically defined RSV infection.
Fold-change in serum anti-p27 antibody titer between study visits is shown. Pairwise comparisons in post-estimated GMNAT between visits within each group was tested and considered significant at p <0.05.
3.4. P27LA in healthy adults
As for IgA and IgG anti-p27 antibodies, all adults had detectable P27LA levels for the total duration of the study period (Table 3). P27LA GMC remained stable in the uninfected group for the entire study period, the GMC significantly increased in the acutely infected group between all study visits, and the GMC decreased over the season in the recently infected group, though not statistically significant. It is important to note that unlike anti-p27 ELISAs (IgG and IgA) that measure antibodies that directly binds to p27 peptide, the competitive P27LA assay measures antibodies that inhibit binding of p27 either directly or by steric hindrance.
Table 3.
P27-like antibody (P27LA) concentrations in sera from healthy adults over an RSV season
| P27LA | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| GMC | Fold Change | ||||||||
| Infection Status | Visit 1 | Visit 2 | Visit 3 | Visit 1–2 | p Valueb | Visit 1–3 | p Value | Visit 2–3 | p Value |
| Uninfected | 1424.7 (726.5–2793.8)a | 1294.0 (670.1–2499.2) | 1178.5 (598.0–2322.5) | −1.1 | 0.641 | −1.2 | 0.505 | −1.1 | 0.840 |
| Acutely Infected | 2596.2 (808.9–8332.6) | 4695.8 (2857.1–7717.0) | 5417.9 (3112.2–9432.0) | 1.8 | 0.003 | 2.1 | 0.000 | 1.2 | 0.002 |
| Recent Infection | 4043.8 (2684.3–6091.8) | 1800.4 (1585.3–2044.7) | 2106.8 (1160.0–3826.6) | −2.2 | 0.515 | −1.9 | 0.563 | 1.2 | 0.942 |
Geometric mean concentration (ng/mL) for serum (95% confidence interval) IgG anti-p27 antibodies in healthy adults with or without a serologically defined RSV infection.
Fold-change in serum anti-p27 antibody titer between study visits is shown. Pairwise comparisons in post-estimated GMNAT between visits within each group was tested and considered significant at p <0.05.
3.5. Correlation of p27 antibody and neutralizing antibody titers
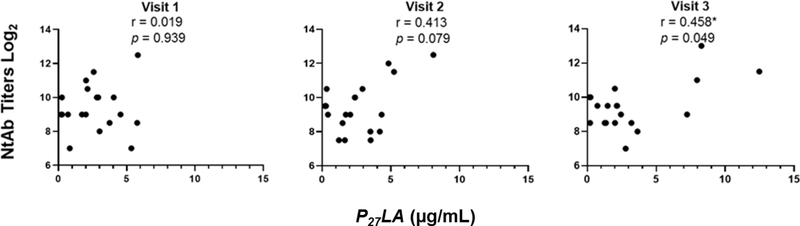
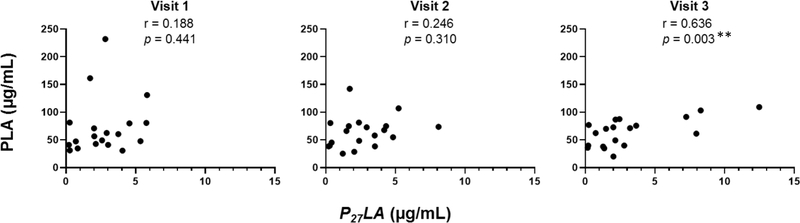
To test whether the kinetics of p27 responses related to known protective antibody responses, we measured correlation to neutralizing antibody and PLA. Statistically significant correlations between IgG anti-p27 or IgA anti-p27 with neutralizing antibody titers to four different RSV strains (prototypic isolates: RSV/A/Tracy and RSV/B/18537; contemporaneous isolates: RSV/A/ON and RSV/B/BA) were not observed. Correlations between P27LA responses and neutralizing antibody titers to RSV/A/Tracy and PLA are shown in Figure 1 and 2, respectively. The correlation values between P27LA and neutralizing antibody increased throughout the study period, with values ranging from 0.187–0.458 by Visit 3, reaching statistical significance with neutralizing antibody titers to RSV/A/Tracy at Visit 3 (Figure 1). In order to compare antibody responses to p27 and other antigenic sites on the F protein, we compared P27LA responses to PLA responses at each study visit (Figure 2). Similar trends in correlation were observed between P27LA responses and PLA as with neutralizing antibody; the correlation increased throughout the study period and P27LA levels were highly correlated with PLA responses by Visit 3 (r = 0.636, p = 0.003).
Fig 1. Correlation of RSV p27 competitive antibody concentrations and neutralizing antibody titers at Visits 1, 2, and 3.
Pearson’s correlation coefficients were calculated to assess the linear association between the neutralizing antibody titer to RSV/A/Tracy and competitive antibody concentrations to p27 peptide at each study visit. n = 19. Significant correlations are indicated (*p ≤ 0.05).
Fig 2. Correlation of RSV p27 competitive antibody concentrations and PLA (site II competitive antibody) concentrations at Visits 1, 2, and 3.
Pearson’s correlation coefficients were calculated to measure the strength of the linear association between the competitive antibody directed to Site II and competitive antibody concentrations to p27 peptide at each study visit. n = 19. Significant correlations are indicated (**p ≤ 0.01).
4. Discussion
All healthy adults enrolled in this study had detectable IgG and IgA anti-p27 serum antibody levels for the entirety of the study duration. These results are consistent with levels of anti-p27 antibody observed previously in adults who underwent a hematopoietic stem cell transplant [26]. Serum IgG anti-p27 antibody levels followed the same three distinct patterns described previously for RSV-specific antibodies targeting other antigenic regions of the F protein [28]. The uninfected group maintained anti-p27 antibody levels for the duration of the study, anti-p27 antibody increased in the acutely infected group, and declined in the recently infected group [28].
It has previously been assumed that p27 is cleaved post-translationally in the trans-Golgi and is absent from infectious virus. However, the observed results from this study that found that all adults have detectable levels of p27-specific antibody, and these also follow the same dynamics displayed by other antigenic site-specific antibody responses following natural infection. Additionally, p27 has been observed both to be expressed on the surface of RSV infected cells and lungs of RSV infected mice [23]. These results support the conclusion of Krzyzaniak et al., [22] by suggesting that p27 is potentially present on the F protein of the infectious viral particles, and therefore could be present to enter by micropinocytosis where the second furin cleavage occurs leading to fully mature F proteins for virus-cell fusion to occur.
In our study, competitive antibody responses to p27 were 19.8–27.3-fold lower than those detected towards antigenic site II (PLA) [28] and comprised only 1% of the total competitive antibody response measured [28]. These results indicate that while antibody was elicited toward p27 peptide after an RSV infection, it was not an immunodominant epitope in healthy adults. Similarly, Fuentes et al [24] observed an age-dependent antibody response toward p27 with children generating a higher p27 antibody response compared to adults. Our study did not include children and thus we were unable to confirm the immunodominant nature of p27 epitope, as previously observed in sera of young children. This reduced magnitude of p27LA could also indicate that the F protein may exist in both partially and fully cleaved forms on the surface of intact virions. This hypothesis is supported by the higher antibody concentrations to site II (and in fact all other antigenic sites [28]), as site II is present regardless of whether F is partially or fully cleaved, and would therefore be present and available in larger quantities on the surface of the virion than p27. This could support that RSV utilizes both proposed models of fusion and viral entry, both at the cell surface and by utilizing micropinocytosis [19–22].
Serum p27 antibody responses were prolonged after acute RSV infection, with significant increases in p27 antibody in the acutely infected group between the second and third study visits, a period of five months. Interestingly, this prolonged increase in antibody after RSV infection was unique in response to p27 as it was not observed toward other F site-specific antibody responses in healthy adults [28]. This prolonged increase in antibody following infection has not been widely-reported previously and the biological significance of this prolonged humoral response is unknown. This prolonged increase may explain why P27LA concentrations best correlated with neutralizing antibody and PLA concentrations later in the study at Visit 3.
Serum IgG anti-RSV/B p27 antibody titers were lower than IgG anti-RSV/A p27 at enrollment and in response to an acute infection. This was unexpected, as RSV/B was the predominant circulating RSV strain in the community during the study period and, thus, the likely etiological agent of the RSV infections in our cohort [28]. This assumption that RSV/B was likely the infecting strain is supported by neutralizing antibody responses measured in these cohorts, where higher fold changes were observed in the infected groups towards RSV/B rather than RSV/A strains [28]. A potential explanation of the observed lower RSV/B p27 titers, is that the p27 mAb used in these assays was developed from an RSV/A strain. To try to guard against this bias, however, we used RSV/A and RSV/B p27 consensus peptides separately for generating standard curves for its respective assay. Another potential explanation for higher RSV/A p27 antibody titers is differences in the antigenicity of the p27 peptide of RSV/A and RSV/B subtype viruses. While differences in RSV subtypes are found primarily in the attachment (G) glycoprotein [33], the p27 domain of the F also contains significant variation between the RSV/A and RSV/B subtypes [10].
Five potential N-linked glycosylation sites are conserved among all RSV isolates having the consensus sequence N-X-T/S, where X can represent any amino acid except proline. One glycosylation site is found within the F1 subunit (N500), two glycosylation sites (N27 and N70) are found within the F2 subunit, and two sites are located within the p27 domain (N116 and N126). An additional potential N-glycosylation site is also found at position N120 within p27, but the availability of this additional site is RSV strain dependent [13]. Analysis of the availability of this third site has been limited to only two RSV/A strains, A2 and Long, however; possible differences in availability of this site between RSV/A and RSV/B subtypes have not been investigated. Variation in the number of potential glycosylation sites could lead to differences in how the F protein is processed, as it is well established that N-glycosylation of viral surface glycoproteins is a prerequisite for proper folding and subsequent trafficking to the plasma membrane [34]. In addition, N-glycan structures could alter receptor binding and/or fusion activity, or they could mask antigenic sites, leading to altered antibody recognition [27, 35]. Because N120 is close to both polybasic cleavage sites, it is tempting to speculate that the attachment of an additional N-linked glycosylation could lead to alterations in efficacy of furin-like protease-mediated cleavage and, thus, whether both furin cleavages occur in the trans-Golgi or whether only a single cleavage event takes place. As both the RSV/A and RSV/B consensus p27 peptides were synthetically produced peptides, neither contained any glycosylation. Follow-up studies testing differences in availability of p27 on RSV/A versus RSV/B virions or glycosylation could inform our understanding of the way the F protein is processed during maturation.
Additionally, the vaccine candidate developed by Novavax, which recently underwent phase III evaluation for protection of infants through maternal immunization, includes p27 in its formulation [36]. This candidate nanoparticle RSV-F vaccine has been shown to be incredibly effective in preclinical models and induces antibody responses to p27 epitope [37,38]. While it did not meet its primary outcome in phase III trials, it did meet several secondary outcomes and likely will undergo further development [36]. One possibility raised from Novavax’s nanoparticle RSV-F vaccine is that retaining p27 within their F nanoparticle adds stability to the F protein that allows for uniform expression and clustering into distinct rosettes. As expression, purification, and stability of the F protein is an important hurdle in RSV vaccine development currently, this last point warrants future consideration.
There are several limitations in our study. First, the study had a small sample size. Nonetheless, antibody responses to antigenic site p27 were consistent with those measured for other F site-specific responses in the same cohort. Second, this study focused solely on serum p27 antibody responses. Differences in mucosal IgA anti-p27 antibody responses, such as observed by Ye et al [26] may be important in controlling RSV infection. Additionally, recent evidence suggests that p27-specific antibody responses mediate protection against RSV challenge through alternative functions to neutralizing potency, particularly antibody-dependent cellular cytotoxicity. Therefore, evaluation of these alternative functions will be important to evaluate in future studies. Finally, p27LA was only assessed for RSV/A and not RSV/B. As subtype-specific differences in responses were observed in p27 IgG responses, it will be important to analyze these distinctions in the future.
In conclusion, p27 is being recognized by the immune system of healthy adults who generate a response to this antigen following natural infection. The context of how this peptide is being recognized, either as a free peptide, a partially cleaved peptide bound to the F protein, or both remains to be answered. As this epitope is recognized during an acute infection, further studies of the biological role of p27 in immunity and in the maturation of the F protein are warranted.
Highlights.
Serum p27 antibody was measured following natural RSV infection in healthy adults
Anti-p27 antibodies were detected in all healthy adults at all study visits
Antibodies to the p27 region are generated following natural RSV reinfection
p27 is not an immunodominant epitope in healthy adults
Serum IgG anti-RSV/B p27 antibody titers were lower than IgG anti-RSV/A p27 titers
Acknowledgements
The authors thank (Dr. Gale Smith) and Novavax (Bethesda, MD) for providing the p27 monoclonal antibody used in assay development.
Funding
Funding was from discretionary funds from PAP and BEG, and NIH grant R01GM115501 to LZ.
Footnotes
Potential conflicts of interest
All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Shi T, McAllister DA, O’Brien KL, Simoes EAF, Madhi SA, Gessner BD, et al. RSV Global Epidemiology Network. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet. 2017. Sep 2;390(10098):946–958. doi: 10.1016/S0140-6736(17)30938-8. Epub 2017 Jul 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hall CB, Weinberg GA, Blumkin AK, Edwards KM, Staat MA, Schultz AF et al. Respiratory syncytial virus-associated hospitalizations among children less than 24 months of age. Pediatrics. 2013. Aug;132(2):e341–8. doi: 10.1542/peds.2013-0303. Epub 2013 Jul 22. [DOI] [PubMed] [Google Scholar]
- [3].Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010. May 1;375(9725):1545–55. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986. Jun;140(6):543–6. doi: 10.1001/archpedi.1986.02140200053026. [DOI] [PubMed] [Google Scholar]
- [5].Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005. Apr 28;352(17):1749–59. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- [6].WHO Vaccine Pipeline Tracker. Available online: https://docs.google.com/spreadsheets/u/1/d/19otvINcayJURCMg76xWO4KvuyedYbMZDcXqbyJGdcZM/pubhtml# (accessed December 8, 2020).
- [7].Borchers AT, Chang C, Gershwin ME, Gershwin LJ. Respiratory syncytial virus--a comprehensive review. Clin Rev Allergy Immunol. 2013. Dec;45(3):331–79. doi: 10.1007/s12016-013-8368-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Karron RA, Buonagurio DA, Georgiu AF, Whitehead SS, Adamus JE, Clements-Mann ML, et al. Respiratory syncytial virus (RSV) SH and G proteins are not essential for viral replication in vitro: clinical evaluation and molecular characterization of a cold-passaged, attenuated RSV subgroup B mutant. Proc Natl Acad Sci U S A. 1997. Dec 9;94(25):13961–6. doi: 10.1073/pnas.94.25.13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ngwuta JO, Chen M, Modjarrad K, Joyce MG, Kanekiyo M, Kumar A, et al. Prefusion F-specific antibodies determine the magnitude of RSV neutralizing activity in human sera. Sci Transl Med. 2015. Oct 14;7(309):309ra162. doi: 10.1126/scitranslmed.aac4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hause AM, Henke DM, Avadhanula V, Shaw CA, Tapia LI, Piedra PA. Sequence variability of the respiratory syncytial virus (RSV) fusion gene among contemporary and historical genotypes of RSV/A and RSV/B. PLoS One. 2017. Apr 17;12(4):e0175792. Erratum in: PLoS One. 2017 Jun 28;12 (6):e0180623. doi: 10.1371/journal.pone.0175792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Tan L, Coenjaerts FE, Houspie L, Viveen MC, van Bleek GM, Wiertz EJ et al. The comparative genomics of human respiratory syncytial virus subgroups A and B: genetic variability and molecular evolutionary dynamics. J Virol. 2013. Jul;87(14):8213–26. doi: 10.1128/JVI.03278-12. Epub 2013 May 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zimmer G, Budz L, Herrler G. Proteolytic activation of respiratory syncytial virus fusion protein. Cleavage at two furin consensus sequences. J Biol Chem. 2001. Aug 24;276(34):31642–50. doi: 10.1074/jbc.M102633200. Epub 2001 Jun 19. [DOI] [PubMed] [Google Scholar]
- [13].Zimmer G, Trotz I, Herrler G. N-glycans of F protein differentially affect fusion activity of human respiratory syncytial virus. J Virol. 2001. May;75(10):4744–51. doi: 10.1128/JVI.75.10.4744-4751.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].González-Reyes L, Ruiz-Argüello MB, García-Barreno B, Calder L, López JA, Albar JP. Cleavage of the human respiratory syncytial virus fusion protein at two distinct sites is required for activation of membrane fusion. Proc Natl Acad Sci U S A. 2001. Aug 14;98(17):9859–64. doi: 10.1073/pnas.151098198. Epub 2001 Aug 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Srinivasakumar N, Ogra PL, Flanagan TD. Characteristics of fusion of respiratory syncytial virus with HEp-2 cells as measured by R18 fluorescence dequenching assay. J Virol. 1991. Aug;65(8):4063–9. doi: 10.1128/JVI.65.8.4063-4069.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bolt G, Pedersen LO, Birkeslund HH. Cleavage of the respiratory syncytial virus fusion protein is required for its surface expression: role of furin. Virus Res. 2000. Jun;68(1):25–33. doi: 10.1016/s0168-1702(00)00149-0. [DOI] [PubMed] [Google Scholar]
- [17].Collins PL, Mottet G. Post-translational processing and oligomerization of the fusion glycoprotein of human respiratory syncytial virus. J Gen Virol. 1991. Dec;72 ( Pt 12):3095–101. doi: 10.1099/0022-1317-72-12-3095. [DOI] [PubMed] [Google Scholar]
- [18].McLellan JS, Chen M, Leung S, Graepel KW, Du X, Yang Y et al. Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science. 2013. May 31;340(6136):1113–7. doi: 10.1126/science.1234914. Epub 2013 Apr 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kahn JS, Schnell MJ, Buonocore L, Rose JK. Recombinant vesicular stomatitis virus expressing respiratory syncytial virus (RSV) glycoproteins: RSV fusion protein can mediate infection and cell fusion. Virology. 1999. Feb 1;254(1):81–91. doi: 10.1006/viro.1998.9535. [DOI] [PubMed] [Google Scholar]
- [20].Kolokoltsov AA, Deniger D, Fleming EH, Roberts NJ Jr, Karpilow JM, Davey RA. Small interfering RNA profiling reveals key role of clathrin-mediated endocytosis and early endosome formation for infection by respiratory syncytial virus. J Virol. 2007. Jul;81(14):7786–800. doi: 10.1128/JVI.02780-06. Epub 2007 May 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].San-Juan-Vergara H, Sampayo-Escobar V, Reyes N, Cha B, Pacheco-Lugo L, Wong T. Cholesterol-rich microdomains as docking platforms for respiratory syncytial virus in normal human bronchial epithelial cells. J Virol. 2012. Feb;86(3):1832–43. Epub 2011 Nov 16. Erratum in: J Virol. 2012 May;86(9):5408. doi: 10.1128/JVI.06274-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Krzyzaniak MA, Zumstein MT, Gerez JA, Picotti P, Helenius A. Host cell entry of respiratory syncytial virus involves macropinocytosis followed by proteolytic activation of the F protein. PLoS Pathog. 2013;9(4):e1003309. doi: 10.1371/journal.ppat.1003309. Epub 2013 Apr 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lee J, Lee Y, Klenow L, Coyle EM, Tang J, Ravichandran S, Golding H, Khurana S. Protective antigenic sites identified in respiratory syncytial virus fusion protein reveals importance of p27 domain. EMBO Mol Med. 2021. Nov 8:e13847. doi: 10.15252/emmm.202013847. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Fuentes S, Coyle EM, Beeler J, Golding H, Khurana S. Antigenic Fingerprinting following Primary RSV Infection in Young Children Identifies Novel Antigenic Sites and Reveals Unlinked Evolution of Human Antibody Repertoires to Fusion and Attachment Glycoproteins. PLoS Pathog. 2016. Apr 21;12(4):e1005554. doi: 10.1371/journal.ppat.1005554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Fuentes S, Hahn M, Chilcote K, Chemaly RF, Shah DP, Ye X et al. Antigenic Fingerprinting of Respiratory Syncytial Virus (RSV)-A-Infected Hematopoietic Cell Transplant Recipients Reveals Importance of Mucosal Anti-RSV G Antibodies in Control of RSV Infection in Humans. J Infect Dis. 2020. Feb 3;221(4):636–646. doi: 10.1093/infdis/jiz608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ye X, Cabral de Rezende W, Iwuchukwu OP, Avadhanula V, Ferlic-Stark LL, Patel KD et al. Antibody Response to the Furin Cleavable Twenty-Seven Amino Acid Peptide (p27) of the Fusion Protein in Respiratory Syncytial Virus (RSV) Infected Adult Hematopoietic Cell Transplant Recipients. Vaccines (Basel). 2020. Apr 21;8(2):192. doi: 10.3390/vaccines8020192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Leemans A, Boeren M, Van der Gucht W, Pintelon I, Roose K, Schepens B et al. Removal of the N-Glycosylation Sequon at Position N116 Located in p27 of the Respiratory Syncytial Virus Fusion Protein Elicits Enhanced Antibody Responses after DNA Immunization. Viruses. 2018. Aug 14;10(8):426. doi: 10.3390/v10080426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Blunck BN, Aideyan L, Ye X, Avadhanula V, Ferlic-Stark L, Zechiedrich L et al. A prospective surveillance study on the kinetics of the humoral immune response to the respiratory syncytial virus fusion protein in adults in Houston, Texas. Vaccine 2021. Feb 22;39(8):1248–1256. doi: 10.1016/j.vaccine.2021.01.045. Epub 2021 Jan 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Piedra PA, Grace S, Jewell A, Spinelli S, Bunting D, Hogerman DA et al. Purified fusion protein vaccine protects against lower respiratory tract illness during respiratory syncytial virus season in children with cystic fibrosis. Pediatr Infect Dis J. 1996; 15(1):23–31. doi: 10.1097/00006454-199601000-00006. [DOI] [PubMed] [Google Scholar]
- [30].Singh A, Maichle R, Lee SE. On the computation of a 95% upper confidence limit of the unknown population mean based upon data sets with below detection limit observations. U.S. Environmental Protection Agency EPA/600/R-06/022, 2006. doi: 10.3389/fimmu.2019.00706. [DOI] [Google Scholar]
- [31].Ye X, Iwuchukwu OP, Avadhanula V, Aideyan LO, McBride TJ, Ferlic-Stark LL, Patel KD, Piedra FA, Shah DP, Chemaly RF, Piedra PA. Comparison of Palivizumab-Like Antibody Binding to Different Conformations of the RSV F Protein in RSV-Infected Adult Hematopoietic Cell Transplant Recipients. J Infect Dis. 2018. Mar 28;217(8):1247–1256. doi: 10.1093/infdis/jiy026. [DOI] [PubMed] [Google Scholar]
- [32].Ye X, Iwuchukwu OP, Avadhanula V, Aideyan LO, McBride TJ, Ferlic-Stark LL, Patel KD, Piedra FA, Shah DP, Chemaly RF, Piedra PA. Antigenic Site-Specific Competitive Antibody Responses to the Fusion Protein of Respiratory Syncytial Virus Were Associated With Viral Clearance in Hematopoietic Cell Transplantation Adults. Front Immunol. 2019. Mar 29;10:706. doi: 10.3389/fimmu.2019.00706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Walsh EE, Hall CB, Schlesinger JJ, Brandriss MW, Hildreth S, Paradiso P. Comparison of antigenic sites of subtype-specific respiratory syncytial virus attachment proteins. J Gen Virol. 1989. Nov;70 ( Pt 11):2953–61. doi: 10.1099/0022-1317-70-11-2953. [DOI] [PubMed] [Google Scholar]
- [34].Varki A. Biological roles of glycans. Glycobiology. 2017. Jan;27(1):3–49. doi: 10.1093/glycob/cww086. Epub 2016 Aug 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Vigerust DJ, Shepherd VL. Virus glycosylation: role in virulence and immune interactions. Trends Microbiol. 2007. May;15(5):211–8. doi: 10.1016/j.tim.2007.03.003. Epub 2007 Mar 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Madhi SA, Polack FP, Piedra PA, Munoz FM, Trenholme AA, Simões EAF, et al. Prepare Study Group. Respiratory Syncytial Virus Vaccination during Pregnancy and Effects in Infants. N Engl J Med. 2020. Jul 30;383(5):426–439. doi: 10.1056/NEJMoa1908380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Patel N, Tian JH, Flores R, Jacobson K, Walker M, Portnoff A, et al. Flexible RSV Prefusogenic Fusion Glycoprotein Exposes Multiple Neutralizing Epitopes that May Collectively Contribute to Protective Immunity. Vaccines (Basel). 2020. Oct 14;8(4):607. doi: 10.3390/vaccines8040607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Patel N, Massare MJ, Tian JH, Guebre-Xabier M, Lu H, Zhou H, et al. Respiratory syncytial virus prefusogenic fusion (F) protein nanoparticle vaccine: Structure, antigenic profile, immunogenicity, and protection. Vaccine. 2019. Sep 24;37(41):6112–6124. doi: 10.1016/j.vaccine.2019.07.089. Epub 2019 Aug 12. [DOI] [PubMed] [Google Scholar]