Abstract
Background
Tuberculosis (TB) remains a major global health problem, in the top 10 causes of death. As a regulator of the immune response, T-helper (Th) cells activate other lymphocytes from the immune system, such as B cells, to destroy the TB pathogen by releasing CD4 and CD8 Th cells. Diabetes mellitus (DM) is a known cause of developing active pulmonary TB. Few studies have examined the biomolecular expression affecting Mycobacterium tuberculosis (MTB) and multidrug-resistant (MDR) MTB, which are associated with low immunity represented by TB in diabetes and CD4 and CD8 levels.
Materials and methods
This animal study used a post-test control group design. We performed an experimental study using 30 BALB/c mice, each weighing 25 g. It included six experimental animal groups, of which three had a diabetes condition induced using intraperitoneal streptozotocin, and all were infected with MTB or MDR TB. We evaluated the CD4 and CD8 levels in each group and analyzed the differences.
Results
We found a significant difference in CD4 and CD8 levels in MTB and MDR TB conditions.
Conclusion
This study shows that acute infection in experimental mice with MTB and MDR TB with or without diabetes had the highest levels of both CD4 and CD8 cells, which can be a sign of increased cellular immunity in a mice model.
Keywords: Mycobacterium tuberculosis, Multidrug resistance, CD4, CD8, Diabetes mellitus
Highlights
-
•
Tuberculosis (TB) is still a major global health problem.
-
•
TB with comorbid diabetes mellitus (DM) are associated with increased CD4 and CD8.
-
•
CD4 and CD8 values are increased in animals with DM plus TB infection.
1. Introduction
Tuberculosis (TB) remains a major health problem, among the top 10 causes of death globally [1,2]. Based on data from the Global TB Report in 2018, the incidence of multidrug-resistant (MDR) TB was 23,000 cases, or the equivalent of 8.8 cases per 100,000 population [3]. New MDR TB cases comprised 2.8%, and MDR TB cases based on re-treatment as much as 16% [4]. The death rate caused by TB with negative HIV status was 107 cases per 264,000 population, and for TB with positive HIV status, it was 9.4 cases per 264,000 population [5]. The incidence of MDR TB at Persahabatan Hospital during 2005–2007 was 14.9% among 3727 pulmonary TB patients. A total of 10,478 patients had suspected MDR TB in 2009–2016, of whom 1509 were diagnosed with it.
As a regulator of the immune response, T-helper (Th) cells activate other lymphocytes from the immune system, such as B cells [6,7]. Th cell activation requires two signals: the first from the binding of antigen receptors on the surface of T cells with the MHC class II antigen complex on APC cells and the second derived from interleukin (IL)-1 (a soluble protein produced by APC cells) [[8], [9], [10]]. These two signals simultaneously increase the receptors or surface expression of lymphokines and IL-2 and growth and differentiation factors for B cells and macrophages, among others. Th cells also activate Tc cells, whose main function is to kill all non-self cells [10]. Tc cells can be distinguished from Th cells because they have CD8 antigens and can recognize foreign antigens with MHC class I profiles. CD4 proteins bind to MHC class II molecules, and CD8 proteins bind MHC class I molecules to APCs. Thus, both CD4 and CD8 cells participate in creating the MHC–antigen complex. Activated Tc cells produce cytokines that can destroy pathogens [10,11].
MDR TB is resistant to INH and rifampicin [12,13]. The incidence of MDR TB is increasing along with the increasing number of TB incidents in TB patients with an impaired immune system [[14], [15], [16]]. MDR TB can be caused by exposure to MDR TB bacteria or incomplete treatment in ordinary TB cases due to inappropriate previous treatment regimens, inadequate dosage or treatment time, patient non-compliance with medication, or insufficient drug supply [[16], [17], [18]].
Diabetes mellitus (DM) has been a cause of active pulmonary TB development [14,19,20]. Chronic hyperglycemia caused by absolute or relative insulin deficiency disrupts metabolism, causing microvascular and neurological complications [21,22]. These complications increase the risk of infections, including lung infections [22,23]. The functions of neutrophils and macrophages are impaired in the immune system of DM patients, such as chemotaxis, adherence, phagocytosis, and the ability to kill phagocytosed microorganisms [24,25]. Tuberculosis with comorbid DM has been associated with increased baseline frequency and antigen-specific CD4 subsets. CD4 cells are thought to play an important role in memory response to Mycobacterium tuberculosis infection and contribute to pathology [26,27].
Few studies have explained the biomolecular expression that affects the MDR TB condition associated with lower immunity in diabetes. This study aimed to evaluate the differences in CD4 and CD8 levels with MTB and MDR TB infection during the DM condition.
2. Methods
This animal study used a post-test control group design. It was performed in the Laboratory of Molecular Biology and Immunology, Faculty of Medicine, Hasanuddin University, Makassar. The study was conducted after obtaining approval from the animal research ethics committee of Hasanuddin University (code number: 160/UN4.6.4.5.31/PP36/2021). These experimental animals were fed standard chow without additional feeding for approximately one week for the adaptation period. Feeding took place daily, with Aquadest provided as a drink. The cage was standard-shaped, cleaned regularly, and given lighting (12 h light/dark photoperiod) [28,29]. The laboratory animals were treated according to National Institutes of Health guidelines, and this work was carried out in line with the ARRIVE Guidelines for Reporting Animal Research [[30], [31], [32]].
2.1. Population and sample
The subjects selected had met the inclusion criteria: adult female BALB/c mice, each weighing approximately 25 g and 6–8 weeks old. The exclusion criteria in this study were experimental animals who were allergic to injection of streptozotocin (STZ) and animals that died before all blood test subjects were taken. All mice were obtained from Hasanuddin University Animal Laboratory. A total of 30 animals were used in this study, with five in each group (Federer formula). The animals were divided into six groups: the negative control group (without diabetes and without locally injected Mycobacterium tuberculosis/MTB or MDR TB), treatment group without diabetes (subcutaneous locally injected TB bacteria), treatment group without diabetes (subcutaneous locally injected MDR TB bacteria), treatment group with diabetes (without subcutaneous locally injected MTB or MDR TB bacteria), treatment group with diabetes (subcutaneous locally injected MTB bacteria), and treatment group with diabetes (subcutaneous locally injected MDR TB bacteria). Sacrifice was performed 15 days after treatment. Serum was collected to assess CD4 and CD8 expression.
2.2. Induction of type I DM with streptozotocin
Adaptation was performed to induce the condition of DM in experimental animals with an intraperitoneal injection of STZ 40 mg/kg body weight, after 3 days of blood sugar level evaluation. When fasting blood sugar was above 126 mg/dL, the mice were included in the diabetes group [[33], [34], [35]].
2.3. Infection of mice
The animals were infected by intraperitoneal injection of 0.2 mL of MTB or MDR TB bacteria. Blood sugar levels and bacterial profiles were evaluated on day 14 after the administration of STZ and MTB bacteria in each treatment group. Sacrifice was performed 15 days after treatment by induction of CO2 asphyxia.
2.4. CD4 and CD8 expression assessment
Before sacrifice, blood from all animals was collected using a 3-mL 25G needle syringe, followed by injection of formalin. Then, 10–20 cc of peripheral blood was diluted with phosphate buffer saline (PBS; pH 7.4) at a ratio of 1:2, placed in layers on Ficoll-Paque Plus medium, and centrifuged at 4 °C for 30 min. PBMC was taken from the interphase layer between the Ficoll-Paque and serum, then washed twice with PBS. CD4⁺ T cells were isolated from the PBMC by negative sorting, using a streptavidin mixture conjugated with CD8 antibodies. The PBMC was incubated with the antibody mixture at room temperature for 15 min and magnetically combined with monoclonal antibodies at room temperature for 30 min (Human T Lymphocyte Separation Kit). CD4⁺ T cells were purified using magnetic boards with >90–95% purity, rated by CD4⁺ T lymphocyte counter cytometry flow.
CD4 and CD8 T cells from the PBMC (1 × 10 cells) were colored with a combination of monoclonal antibodies: FITC-labelled anti-CD4, phycoerythrin (PE)-labelled anti-CD25, and PE-cy7-labelled anti-CCR4. The cells were then washed twice with cold flow cytometry staining buffer (0.5% BSA, 0.1% sodium azide, 2 mM EDTA).
To determine the migration capacity of Treg, chemotaxis testing was conducted in 24-well chemotaxis chambers with polyvinyl pyrrolidine-free polycarbonate membranes (pore size 5 μm). At the base of the chamber, each well was filled with 600 μL of agonists at the appropriate concentration (diluted at 1640 RPM and 0.1% BSA) and carefully coated over the polycarbonate membrane. Human chemokines were used: up to 100 ng/mL TARC (CCL17) and up to 50 ng/mL MDC (CCL22). The sorted CD4⁺ T cells were washed twice and suspended at 1640 RPM medium and 0.1% BSA at 5 × 100 h/mL, and 100 μL cell suspensions were added at the top of the chamber. The chamber was incubated for 2 h with a humidity of 5% CO₂ at 37 °C, and the migration of cells through the membrane to the bottom of the chamber was calculated by FACScan for 60 s at a rate of 60 μL/min.
2.5. Statistical analysis
The data were divided by type and presented as graphs and tables. The analysis used SPSS version 25 (IBM Corp.). Differences in CD4 and CD8 expression in immunocompetent sick subjects compared to sick subjects were analyzed with independent t-tests. Data differences between sick subjects and controls were analyzed by analysis of variance (ANOVA). Statistical significance was determined at P < 0.05.
3. Results
3.1. Characteristics of MTB and MDR TB experimental animals with diabetes conditions
Table 1 shows the bacterial level per field of view of all groups. Most of the mice with diabetes had a higher bacterial level. The highest mean value was in the DM MDR TB group (201 ± 23.52), followed by the DM MTB (197.60 ± 14.31), MDR TB 181.60 (±13.93), and MTB (174 ± 15.95) groups. A statistically significant difference existed in the bacterial level of the DM MTB MDR group compared to other groups. The condition of diabetes triggered a higher bacterial level. A significant difference in bacterial level was found in all treatment groups (P = 0.00).
Table 1.
Basic characteristics of diabetic and control experimental animals.
| MTB level/field view | Mean | SD | Min–Max | Median | P |
|---|---|---|---|---|---|
| Negative control | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 |
| MTB | 174.00 | 15.95 | 153–197 | 174 | |
| MDR TB | 181.60 | 13.93 | 169–204 | 178 | |
| Negative control DM | 0.00 | 0.00 | 0.00 | 0.00 | |
| DM MTB | 197.60 | 14.31 | 183–217 | 195 | |
| DM MDR TB | 201.00 | 23.52 | 174–237 | 198 |
3.2. Comparison of CD4 expression level in MDR TB with DM compared to controls
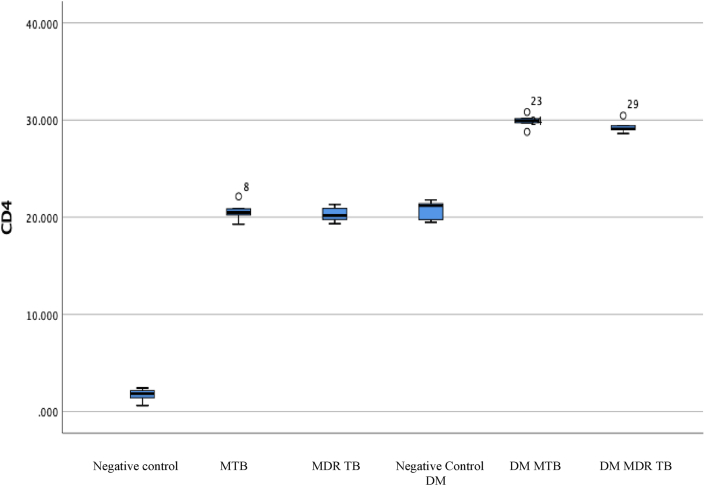
CD4 expression was analyzed in the treatment group of diabetic mice with MTB and MDR TB infection. The evaluation was carried out for each group of experimental animals, and the CD4 values are shown in Fig. 1.
Fig. 1.
CD4 level in the MTB diabetes, MDR TB, and control groups. The highest expression values were obtained in the DM MTB and DM MDR TB groups.
Table 2 shows the CD4 level in each group. The negative control group had a mean CD4 level of 1.69 (±0.70), whereas the MTB group had a higher level of 20.59 (±1.04) followed by the MDR TB (20.30 ± 0.81) and negative control DM (20.71 ± 1.02) groups. The highest levels were in the DM MTB (29.88 ± 0.74) and DM MDR TB (29.32 ± 0.69) groups. The ANOVA found significant differences between the mean CD4 level of all groups (P = 0.00). The highest CD4 levels were in the MTB and MDR TB with diabetes condition groups.
Table 2.
CD4 comparison of the MDR TB and diabetes treatment groups versus controls.
| CD4 level | Mean | SD | Min–Max | P |
|---|---|---|---|---|
| Negative control | 1.69 | 0.70 | 0.622–2.42 | 0.00 |
| MTB | 20.59 | 1.04 | 19.27–22.14 | |
| MDR TB | 20.30 | 0.81 | 19.33–21.33 | |
| Negative control DM | 20.71 | 1.02 | 19.48–21.76 | |
| DM MTB | 29.88 | 0.74 | 28.78–30.82 | |
| DM MDR TB | 29.32 | 0.69 | 28.62–30.45 |
Based on the post hoc LSD analysis, Table 3 shows significant differences between all treatment groups, except for the negative control group DM vs. DM MDR TB, MTB vs. MDR TB, MDR TB vs. negative controls DM, and DM MTB vs. DM MDR TB (P > 0.05). The most significant differences were in the CD4 negative control vs. DM MTB groups and the negative control vs. DM MDR TB groups.
Table 3.
Post hoc test of CD4 differences between groups.
| Animal group | Mean difference | P |
|---|---|---|
| Negative control vs. MTB | −18.90 | 0.00 |
| Negative control vs. MDR TB | −18.60 | 0.00 |
| Negative control vs. negative control DM | −19.02 | 0.00 |
| Negative control vs. DM MTB | −28.18 | 0.00 |
| Negative control vs. DM MDR TB | −27.63 | 0.00 |
| Negative control DM vs. DM MTB | −9.16 | 0.830 |
| Negative control DM vs. DM MDR TB | −8.60 | 0.00 |
| MTB vs. MDR TB | 0.29 | 0.587 |
| MTB vs. negative control DM | −0.11 | 0.00 |
| MTB vs. DM MTB | −9.28 | 0.00 |
| MTB vs. DM MDR TB | −8.72 | 0.00 |
| MDR TB vs. negative control DM | −0.41 | 0.450 |
| MDR TB vs. DM MTB | 9.57 | 0.00 |
| MDR TB vs. DM MDR TB | −9.03 | 0.00 |
| DM MTB vs. DM MDR TB | 0.55 | 0.312 |
3.3. Comparison of CD8 expression in MDR TB with DM compared to controls
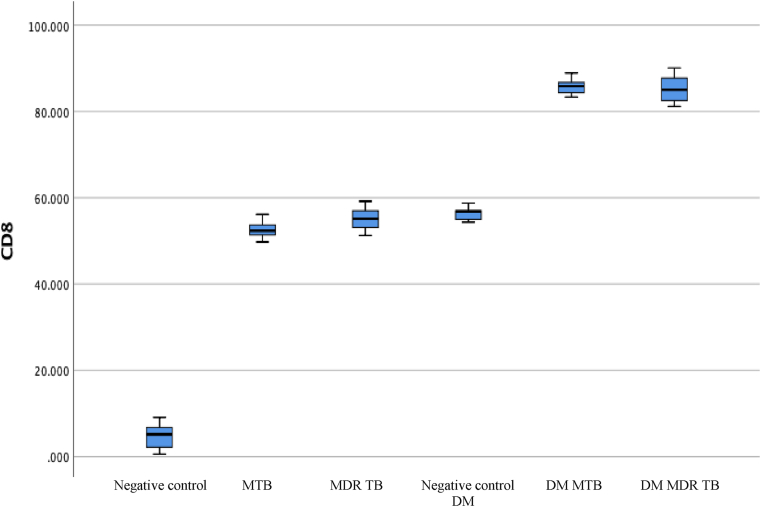
CD8 expression was analyzed for the diabetic animal groups infected by MTB and MDR TB. Evaluation was carried out for each experimental animal group. The CD8 values are shown in Fig. 2 and Table 4.
Fig. 2.
CD8 level in the diabetic MTB, MDR TB, and control groups. The highest expression value was obtained in the DM MTB and DM MDR TB groups.
Table 4.
Comparison of CD8 cells in the MDR TB and diabetes treatment groups versus controls.
| CD8 level | Mean | SD | Min–Max | P |
|---|---|---|---|---|
| Negative control | 4.74 | 3.43 | 0.54–9.07 | 0.00 |
| MTB | 49.67 | 2.42 | 49.75–56.15 | |
| MDR TB | 55.13 | 3.10 | 51.27–59.16 | |
| DM negative control | 56.37 | 1.75 | 54.34–58.77 | |
| DM MTB | 85.84 | 2.14 | 83.35–88.90 | |
| DM MDR TB | 85.30 | 3.65 | 81.17–90.07 |
Table 4 shows the CD8 level for each group. The negative control CD8 value was 4.74 (±3.43), whereas in animals with MTB infection, it increased to 49.67 (±2.42). The value was 55.13 (±3.10) for MDR TB, 56.37 (±1.75) for DM negative control, and the highest in the DM MTB (85.84 ± 2.14) and DM MDR TB (85.30 ± 3.65) groups. A significant difference was found in the CD8 level between groups (P = 0.00).
Table 5 shows the post hoc analysis results. The most significant difference in the mean CD8 level was for negative control vs. DM MDR TB, followed by negative control vs. DM MDR TB.
Table 5.
Post hoc analysis of CD8 level comparison between groups.
| Animal group | Mean difference | P |
|---|---|---|
| Negative control vs. MTB | −47.92 | 0.00 |
| Negative control vs. MDR TB | −50.38 | 0.00 |
| Negative control vs. negative control DM | −51.63 | 0.00 |
| Negative control vs. DM MTB | −81.09 | 0.00 |
| Negative control vs. DM MDR TB | −80.56 | 0.00 |
| Negative control DM vs. DM MTB | −29.46 | 00.00 |
| Negative control DM vs. DM MDR TB | −28.92 | 0.00 |
| MTB vs. MDR TB | −2.46 | 0.183 |
| MTB vs. negative control DM | −3.70 | 0.050 |
| MTB vs. DM MTB | −33.17 | 0.00 |
| MTB vs. DM MDR TB | −32.63 | 0.00 |
| MDR TB vs. negative control DM | −1.24 | 0.495 |
| MDR TB vs. DM MTB | −30.70 | 0.00 |
| MDR TB vs. DM MDR TB | −30.17 | 0.00 |
| DM MTB vs. DM MDR TB | 0.53 | 0.767 |
4. Discussion
Diabetes significantly increased the bacterial level in both MTB and MDR TB infection. Previously, diabetes has been known as a risk factor for TB and related high mortality [14]. The frequency of TB in DM patients has been reported to be 3–10 times higher than in non-diabetic patients [14,36]. Most DM patients experience increased reactivation of TB lesions and are more susceptible to reinfection [27].
Comorbid factors such as type 2 DM increase the risk of infection with TB, especially MDR TB, because low cellular immunity can increase the reactivation of primary infection and the incidence of drug resistance. A prospective study of older adults showed an increased risk of TB infection in uncontrolled DM with HbA1c levels >7% [37].
Alveolar macrophages have a central role in MTB infection and replication. These macrophages digest bacilli to encase them in phagosomes and fuse with lysosomes, along with bacterial digestion and the production of antimicrobial molecules. Hyperglycemic conditions reduce the expression of signals and chemokines that recruit macrophages, neutrophils, and innate lymphocytes that produce a barrier to the transmigration of leukocytes to the alveolus infected with MTB. The interaction between MTB and macrophage receptors (Toll-like receptors; TLRs) produces chemokines (IL-8, monocyte chemoattractant protein-1 [MCP-1], macrophage inflammatory protein-1 alpha [MIP-1α], and the cytokines interferon-γ [IFN-γ], IL-2, IL-12, IL-18, and tumor necrosis factor-α [TNF-α]), which act as signals of infection. This signal induces the migration of monocytes and dendritic cells from the bloodstream to the infected part of the lung. The dendrite cells are destroyed by CD4 and CD8 T cells [38,39].
CD4 and CD8 also play a role in granulomatous formation for bacterial isolation. Increases in CD4 and CD8 have been associated with active infectious conditions. This is consistent with the findings of this study that acute infection in experimental mice with MTB and MDR TB drove CD4 and CD8 levels significantly higher to kill the bacteria. Acute diabetic conditions trigger more severe inflammation and make CD4 and CD8 levels higher in MTB and MDR TB. The effect of acute changes in glucose metabolism arising from glucose loading has been described by Aika et al., who reported that the proportion of CD4+ T cells increased after glucose loading in diabetes. The reason that the proportion of CD4 T cells changed remains unclear. Several mechanisms, such as changes in thymus output, peripheral proliferation, and altered redistribution, are possible but cannot explain the CD4 cell changes in diabetes and TB infection [40,41].
This study found differences in CD4 and CD8 cell levels due to diabetes. The highest CD4 level was in MTB and MDR TB with diabetes compared to the MTB only, MDR TB without diabetes, and negative control groups. MTB and MDR TB with diabetes also triggered the highest CD8 level compared to the MTB and MDR TB without diabetes and control groups. Whether the immunodeficiency conditions that occur in diabetes can suppress or increase CD4 and CD8 levels remains unclear in both MTB and MDR TB infection. Another report found that the proportion of CD4+ T cells increased and CD8+ T cells decreased after acute hyperglycemia in both the diabetic and non-diabetic population, but whether diabetes and MDR TB trigger CD4 and CD8 changes is unclear. The condition of diabetes may be able to trigger a decreased immune response, but the stress caused by diabetes in acute TB infection encourages greater inflammation, followed by a T cell response that encourages an initial CD4 and CD8 increase. Elisa et al. evaluated CD4 and CD8 levels in response to QuantiFERON TB Gold Plus for latent MTB and reported that CD8 increased as a response to active TB. Longer monitoring of TB infection and MDR TB may thus be required in diabetes [[42], [43], [44]].
A limitation of this study was that it did not evaluate CD4 and CD8 values with therapeutic interventions. CD4 and CD8 evaluation is required with a larger number of samples with wider variables to determine the right diagnostic values including examination in active TB patients.
5. Conclusion
This study shows that acute infection in experimental mice with MTB and MDR TB with or without diabetes had the highest levels of both CD4 and CD8 cells, which can be a sign of increased cellular immunity in a mice model.
Provenance and peer review
Not commissioned, externally peer reviewed.
Conflicts of interest
The authors declare that they have no conflict of interests.
Sources of funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
The study was conducted after obtaining approval from the Ethics Commission of Hasanuddin University, number: 160/UN4.6.4.5.31/PP36/2021.
Author contribution
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Consent
This manuscript does not involve human participants, human data, or human tissue.
Registration of research studies
None.
Guarantor
Heidy Agustin.
Acknowledgment
We acknowledge Muhammad Faruk, M.D, for his help in providing us with the linguistic assistance for this experimental study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amsu.2021.102596.
Contributor Information
Heidy Agustin, Email: heidy_agst@yahoo.com.
Muhammad Nasrum Massi, Email: nasrumm2000@yahoo.com.
Irawati Djaharuddin, Email: irawatymuzakkir@gmail.com.
Agus Dwi Susanto, Email: agus_dr2000@yahoo.com.
Andi Asadul Islam, Email: andiasadul@yahoo.com.
Mochammad Hatta, Email: hattaram@yahoo.com.
Agussalim Bukhari, Email: agussalimbukhari@yahoo.com.
Nur Ahmad Tabri, Email: nurahmad_59@yahoo.co.id.
Arif Santoso, Email: arifs777@gmail.com.
Ilhamjaya Patellongi, Email: ilham_pt@yahoo.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Sandhu G. Tuberculosis: current situation, challenges and overview of its control programs in India. J. Global Infect. Dis. 2011;3:143. doi: 10.4103/0974-777X.81691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chakaya J., Khan M., Ntoumi F., Aklillu E., Fatima R., Mwaba P., Kapata N., Mfinanga S., Hasnain S.E., Katoto P.D.M.C., Bulabula A.N.H., Sam-Agudu N.A., Nachega J.B., Tiberi S., McHugh T.D., Abubakar I., Zumla A. Global tuberculosis report 2020 – reflections on the global TB burden, treatment and prevention efforts. Int. J. Infect. Dis. 2021 doi: 10.1016/j.ijid.2021.02.107. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacNeil A., Glaziou P., Sismanidis C., Date A., Maloney S., Floyd K. Global epidemiology of tuberculosis and progress toward meeting global targets — worldwide. MMWR Morb. Mortal. Wkly. Rep. 2018;69:281–285. doi: 10.15585/mmwr.mm6911a2. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Girum T., Muktar E., Lentiro K., Wondiye H., Shewangizaw M. Epidemiology of multidrug-resistant tuberculosis (MDR-TB) in Ethiopia: a systematic review and meta-analysis of the prevalence, determinants and treatment outcome. Trop. Dis. Travel Med. Vaccines. 2018;4:5. doi: 10.1186/s40794-018-0065-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tamuhla T., Dave J.A., Raubenheimer P., Tiffin N. Diabetes in a TB and HIV-endemic South African population: analysis of a virtual cohort using routine health data. PloS One. 2021;16 doi: 10.1371/journal.pone.0251303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luckheeram R.V., Zhou R., Verma A.D., Xia B. CD4 + T cells: differentiation and functions. Clin. Dev. Immunol. 2012;2012:1–12. doi: 10.1155/2012/925135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bacchetta R. Encycl. Immunobiol. Elsevier; 2016. Immunodysregulation, polyendocrinopathy, and enteropathy, X-linked (IPEX) syndrome; pp. 444–450. [DOI] [Google Scholar]
- 8.Mak T.W., Saunders M.E. The Immune Response. Elsevier; 2006. T cell activation; pp. 373–401. [DOI] [Google Scholar]
- 9.Couture A., Garnier A., Docagne F., Boyer O., Vivien D., Le-Mauff B., Latouche J.-B., Toutirais O. HLA-class II artificial antigen presenting cells in CD4+ T cell-based immunotherapy. Front. Immunol. 2019;10 doi: 10.3389/fimmu.2019.01081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alberts B., Johnson A.D., Lewis J., Morgan D., Raff M., Roberts K., Walter P. Mol. Biol. Cell. fourth ed. Garland Science; New York: 2002. Helper T cells and lymphocyte activation.https://www.ncbi.nlm.nih.gov/books/NBK26827/ [Google Scholar]
- 11.Smith I. Mycobacterium tuberculosis pathogenesis and molecular determinants of virulence. Clin. Microbiol. Rev. 2003;16:463–496. doi: 10.1128/CMR.16.3.463-496.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fitzgerald D.W., Sterling T.R., Haas D.W. Mand. Douglas, Bennett's Princ. Pract. Infect. Dis. Elsevier; 2015. Mycobacterium tuberculosis; pp. 2787–2818. e5. [DOI] [Google Scholar]
- 13.Falzon D., Jaramillo E., Weyer K. Multidrug- and rifampicin-resistant tuberculosis (MDR/RR-TB) : global response. Tuberculosis, European Respiratory Society. 2017:OA1944. doi: 10.1183/1393003.congress-2017.OA1944. [DOI] [Google Scholar]
- 14.Restrepo B.I. Diabetes and Tuberculosis., Microbiol. Spectr. 2016;4 doi: 10.1128/microbiolspec.TNMI7-0023-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park M., Satta G., Kon O.M. An update on multidrug-resistant tuberculosis. Clin. Med. 2019;19:135–139. doi: 10.7861/clinmedicine.19-2-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loddenkemper R., Sagebiel D., Brendel A. Strategies against multidrug-resistant tuberculosis. Eur. Respir. J. 2002;20:66S–77s. doi: 10.1183/09031936.02.00401302. [DOI] [PubMed] [Google Scholar]
- 17.Sotgiu G., Tiberi S., Centis R., D'Ambrosio L., Fuentes Z., Zumla A., Migliori G.B. Applicability of the shorter ‘Bangladesh regimen’ in high multidrug-resistant tuberculosis settings. Int. J. Infect. Dis. 2017;56:190–193. doi: 10.1016/j.ijid.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 18.Llaverias G., Danilo C., Mercier I., Daumer K., Capozza F., Williams T.M., Sotgia F., Lisanti M.P., Frank P.G. Role of cholesterol in the development and progression of breast cancer. Am. J. Pathol. 2011;178:402–412. doi: 10.1016/j.ajpath.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Araia Z.Z., Mesfin A.B., Mebrahtu A.H., Tewelde A.G., Osman R., Tuumzghi H.A. Diabetes mellitus and its associated factors in tuberculosis patients in maekel region, Eritrea: analytical cross-sectional study. Diabetes, Metab. Syndrome Obes. Targets Ther. 2021;14:515–523. doi: 10.2147/DMSO.S293557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dooley K.E., Chaisson R.E. Tuberculosis and diabetes mellitus: convergence of two epidemics. Lancet Infect. Dis. 2009;9:737–746. doi: 10.1016/S1473-3099(09)70282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2009;32:S62–S67. doi: 10.2337/dc09-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chawla A., Chawla R., Jaggi S. Microvasular and macrovascular complications in diabetes mellitus: distinct or continuum? Indian J. Endocrinol. Metab. 2016;20:546. doi: 10.4103/2230-8210.183480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alves C., Casqueiro J., Casqueiro J. Infections in patients with diabetes mellitus: a review of pathogenesis. Indian J. Endocrinol. Metab. 2012;16:27. doi: 10.4103/2230-8210.94253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andrews T., Sullivan K.E. Infections in patients with inherited defects in phagocytic function. Clin. Microbiol. Rev. 2003;16:597–621. doi: 10.1128/CMR.16.4.597-621.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Insuela D., Coutinho D., Martins M., Ferrero M., Carvalho V. Neutrophil function impairment is a host susceptibility factor to bacterial infection in diabetes. Cells Immune Syst., IntechOpen. 2020:1–22. doi: 10.5772/intechopen.86600. [DOI] [Google Scholar]
- 26.Ljubić S., Balachandran A., Pavlic-Renar I., Barada A., Metelko Ž. Pulmonary infections in diabetes mellitus. Diabetol. Croat. 2005;33:115–124. [Google Scholar]
- 27.Wijaya I. Tuberkulosis paru pada penderita diabetes melitus. Cermin Dunia Kedokt. 2015;42:412–417. [Google Scholar]
- 28.Islam A.A., Islam I.C., Faruk M., Prihantono P. Comparison of tumor growth in mice balb/C induced breast cancer cells injected with corticosteroids and black seed oil extract. Indian J. Public Heal. Res. Dev. 2018;9:474. doi: 10.5958/0976-5506.2018.00599.5. [DOI] [Google Scholar]
- 29.Josh F., Soekamto T.H., Adriani J.R., Jonatan B., Mizuno H., Faruk M. The combination of stromal vascular fraction cells and platelet-rich plasma reduces malondialdehyde and nitric oxide levels in deep dermal burn injury. J. Inflamm. Res. 2021;14:3049–3061. doi: 10.2147/JIR.S318055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kilkenny C., Browne W.J., Cuthill I.C., Emerson M., Altman D.G. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8 doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nasution R.A., Islam A.A., Hatta M., Prihantono, Massi Warsinggih M.N., Kaelan C., Bahar B., Nasution K.I., Wangi H., Faruk M. Effectiveness of CAPE in reducing vascular permeability after brain injury. Med. Clínica Práctica. 2021;4:100229. doi: 10.1016/j.mcpsp.2021.100229. [DOI] [Google Scholar]
- 32.Laidding S.R., Josh F., Battung S., Bukhari A., Warsinggih, Patellongi I.J., Massi M.N., Islam A.A., Dososaputro I., Faruk M. Combination of platelet rich plasma and stromal vascular fraction on the level of vascular endothelial growth factor in rat subjects experiencing deep dermal burn injury. Ann. Med. Surg. 2021;64:102254. doi: 10.1016/j.amsu.2021.102254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Furman B.L. Streptozotocin‐induced diabetic models in mice and rats. Curr. Protoc. 2021;1 doi: 10.1002/cpz1.78. [DOI] [PubMed] [Google Scholar]
- 34.Al zaben K. Induction of diabetes mellitus in rats using intraperitoneal streptozotocin: a comparison between 2 strains of rats. Eur. J. Sci. Res. 2009;32:398–402. [Google Scholar]
- 35.Mostafavinia A., Amini A., Ghorishi S.K., Pouriran R., Bayat M. The effects of dosage and the routes of administrations of streptozotocin and alloxan on induction rate of type 1 diabetes mellitus and mortality rate in rats. Lab. Anim. Res. 2016;32:160. doi: 10.5625/lar.2016.32.3.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng C., Hu M., Gao F. Diabetes and pulmonary tuberculosis: a global overview with special focus on the situation in Asian countries with high TB-DM burden. Glob. Health Action. 2017;10:1264702. doi: 10.1080/16549716.2016.1264702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crofton S.J., Chaulet P., Maher D., Grosset J., Harris W., Horne N., Iseman M., Watt B. Guidelines for the management of drug-resistant tuberculosis/by sir john crofton, pierre chaulet and dermot maher. World Health Organization. 1997 https://apps.who.int/iris/handle/10665/63465 WHO/TB/96.210 (Rev.1) [Google Scholar]
- 38.Jordao L., Vieira O.V. Tuberculosis: new aspects of an old disease. Int. J. Cell Biol. 2011;2011:1–13. doi: 10.1155/2011/403623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hatta Farsida M., Patellongi Prihantono I., Shabariyah R., Larasati Laras R.A., Islam A.A., Natzir R., Massi M.N., Hamid F., Bahagia A.D. The correlation of Foxp3 + gene and regulatory T cells with scar BCG formation among children with Tuberculosis. J. Clin. Tuberc. Other Mycobact. Dis. 2020;21:100202. doi: 10.1016/j.jctube.2020.100202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rozot V., Patrizia A., Vigano S., Mazza-Stalder J., Idrizi E., Day C.L., Perreau M., Lazor-Blanchet C., Ohmiti K., Goletti D., Bart P.-A., Hanekom W., Scriba T.J., Nicod L., Pantaleo G., Harari A. Combined use of Mycobacterium tuberculosis-specific CD4 and CD8 T-cell responses is a powerful diagnostic tool of active tuberculosis. Clin. Infect. Dis. 2015;60:432–437. doi: 10.1093/cid/ciu795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miya A., Nakamura A., Miyoshi H., Takano Y., Sunagoya K., Hayasaka K., Shimizu C., Terauchi Y., Atsumi T. Impact of glucose loading on variations in CD4+ and CD8+ T cells in Japanese participants with or without type 2 diabetes. Front. Endocrinol. 2018;9:81. doi: 10.3389/fendo.2018.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Behar S.M. 2013. Antigen-specific CD8+ T cells and protective immunity to tuberculosis; pp. 141–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Commandeur S., Lin M.Y., van Meijgaarden K.E., Friggen A.H., Franken K.L.M.C., Drijfhout J.W., Korsvold G.E., Oftung F., Geluk A., Ottenhoff T.H.M. Double- and monofunctional CD4+ and CD8+ T-cell responses to Mycobacterium tuberculosis DosR antigens and peptides in long-term latently infected individuals. Eur. J. Immunol. 2011;41:2925–2936. doi: 10.1002/eji.201141602. [DOI] [PubMed] [Google Scholar]
- 44.Petruccioli E., Chiacchio T., Pepponi I., Vanini V., Urso R., Cuzzi G., Barcellini L., Palmieri F., Cirillo D.M., Ippolito G., Goletti D. Characterization of the CD4 and CD8 T-cell response in the QuantiFERON-TB Gold plus kit. Int. J. Mycobacteriology. 2016;5:S25–S26. doi: 10.1016/j.ijmyco.2016.09.063. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.