Abstract
Although superficial wounds are often easy to treat for healthy individuals, there are some more severe types of wounds (burns, ulcers, diabetic wounds, etc.) that are a challenge for clinicians. A good therapeutic result is based on the delivery of a treatment at the right time, for the right patient. Our goal was to sum up useful knowledge regarding wound healing and wound treatments, based on creams and hydrogels with various active ingredients. We concluded that both preparations have application in preventing infections and promoting healing, but their efficacy is clearly conditioned by the type, depth, severity of the wound and patient profile. However, due to their superior versatility and capability of maintaining the integrity and functionality of the active ingredient, as well as it is controlled release at site, hydrogels are more suited for incorporating different active ingredients. New wound healing devices can combine smart hydrogel dressings with physical therapies to deliver a more efficient treatment to patients if the indications are appropriate.
Keywords: creams, smart hydrogels, wound classification, wound dressing, wound healing
There are a series of factors (mechanical, chemical, radiation, thermal, metabolic etc.) that can disrupt the skin integrity and/or functions and lead to skin wounds. There are many treatment options that can help restore skin barrier, among which hydrogels and creams.
1. INTRODUCTION
Wounds represent a “silent epidemic” among the world population, with a great social and financial impact, affecting the quality of life of millions of people. While acute wound management in a healthy patient is somewhat more approachable, patients with chronic wounds can represent a real challenge in terms of wound evaluation and management. An analysis of Medicare records showed that ∼8.2 million people suffered from acute or chronic wounds, with costs of up to $96.8 billion.1 In Europe, ~2 million people suffer from chronic wounds, while in the United States ∼2% of the total population are estimated to be affected by chronic wounds.1, 2 Doctors and nurses caring for patients with wounds can have a huge impact at multiple levels, social, economic and personal, so it is important that they constantly improve. Knowledge about skin physiology and function, as well as wound healing mechanisms, can enable healthcare staff to better care for wound suffering patients. This basic information will allow the patient to be correctly and efficiently evaluated so that the best treatment plan can be chosen for him. The ultimate goal is to have the best tools for wound management that will allow medical staff to diagnose, make a prognosis and a personalized treatment plan. Wound healing is a dynamic process and the ability to adapt the treatment plan to changes in the wound site or the patient's environment will have a major impact on patient's recovery and quality of life. Because of these, extensive studies have been carried out on the topic of wound healing processes and wound management devices. Hydrogel wound dressings and creams can improve healing and have a beneficial impact on the final outcome. Smart hydrogels enable real‐time wound monitoring and can be used as a conveyance vehicle for bioactive compounds.3, 4
In this review, we briefly describe the anatomy of the skin, its functions and the physiological mechanisms of acute wound healing and we correlated this knowledge with some abnormal healing processes that lead to chronic wounds. We also present traditional and modern tools for wound evaluation such as clinical evaluation, biomarkers and imaging. We discuss the use of hydrogels for wound dressing and wound monitoring and how their unique properties make them suitable for sustaining healing. Also, we discuss the benefits of using creams and ointments and their role in wound healing and highlighted the role of each type of product in the wound healing process based on their unique properties. Finally, we present new wound healing devices that are based on new technologies such as combining smart hydrogels with physical therapies.
2. SKIN ANATOMY
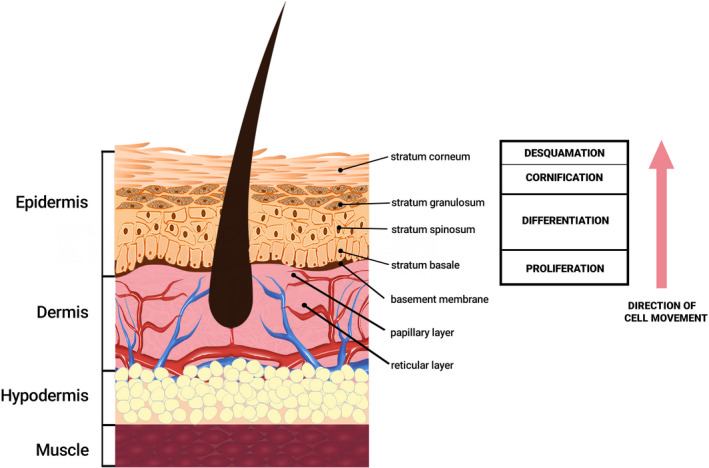
The skin is the largest organ in the human body, being responsible for approximately 16% of total body weight. The two main structural layers that form the skin are the epidermis and the dermis, joined by the basement membrane (Figure 1). Below these layers, there is the subcutaneous tissue—the hypodermis, with the adipose tissue. The epidermis is divided into five layers: the stratum corneum, stratum lucidum, stratum granulosum, stratum spinosum and stratum basale. Stratum lucidum is only found in thick skin. In the classical literature, a sixth layer is also described, derived from the continuous exfoliation of the stratum corneum, the desquamation layer. The dermis consists of two layers: papillary dermis (superficial) and reticular dermis (deep). Regarding the embryonic origin, the epidermis is derived from the ectoderm and is colonized by keratinocytes, melanocytes and Merkel cells originating in the neural crest and Langerhans cells originating in the bone marrow. The dermis and the hypodermis are derived from the mesoderm. It contains fibroblasts, collagen and elastic fibres, proteoglycans and glycosaminoglycans, free and encapsulated nerve endings, Schwann cells, endothelial cells organized in the form of vessels, pericytes, mast cells, tissue macrophages and other cells of the immune system. Skin characteristics and properties (eg thickness, elasticity) vary depending on many parameters (eg age, sex, anatomical location).5, 6, 7
FIGURE 1.
Basic anatomy of skin
The epidermis is an avascular keratinized stratified squamous epithelium in which there are several types of specialized cells, of which keratinocytes are the predominant cells. Keratinocytes are formed from stem cells in the basal layer and as they differentiate, they become corneocytes and form the stratum corneum, a process that lasts between 3 and 6 weeks. They synthesize many proteins, including keratin that has a major structural role in the stratum corneum. Stratum corneum also consists of cell membranes, proteins and complex lipids and provides a major barrier against dehydration and microbial infections. Among the epithelial cells, the basal keratinocytes are the primary cells involved in epidermal healing processes. Langerhans cells are dendritic antigen‐presenting cells, support the local defence of the epidermis and are involved in local immune reactions, for example contact allergies. The epidermis, at the level of the basal layer, is separated from the dermis by the basement membrane (dermo‐epidermal junction), being attached to it by hemidesmosomes. The dermo‐epidermal junction is complex and keratinocytes from the basal layer and components of the extracellular matrix participate in its realization, including the basal lamina but also filaments and anchoring fibrils. The dermo‐epidermal junction must be restored in the wound healing process, otherwise, there is a risk of mechanical separation of the epidermis from the dermis, with the appearance of bullous pathologies (blistering and sloughing).8, 9, 10, 11, 12, 13, 14
At the level of the dermis, which is a dense irregular connective tissue, there is an abundant extracellular matrix, consisting of specialized proteins and carbohydrates. The papillary layer, which begins at the level of the epidermal basement membrane, is mainly populated with fibroblasts and the reticular layer contains mainly thick collagen fibres. The dermis provides metabolic and mechanical support for the epidermis due to its special structure that allows it to maintain its integrity even in case of exposure to intense mechanical stress. If injuries occur, the dermis does not regenerate, at this level a repair process takes place. Under the dermis, there is the hypodermis which is a type of connective tissue mainly consisting of adipocytes. This layer separates the dermis from other structures such as fascia, muscles or bone. In the case of a skin wound, the hypodermis does not regenerate, being replaced by scar tissue rich in collagen.7, 10, 12, 15, 16
3. SKIN FUNCTIONS
Skin functions (Figure 2) are reflected by its structural characteristics. First of all, it is a protective barrier against the harmful environmental factors, with an important role in maintaining the body's homeostasis. The tight intercellular junctions and the stratum corneum form the mechanical barrier. The microbiome, the chemical and the immunological barriers also participate in the fulfilment of the barrier function. Many proteins such as cystatin, desmoplakin and filaggrin contribute to the achievement of this role, while the hydrophobic layer of lipids prevents water loss. The keratinocytes from the spinous layer produce keratohyalin granules and lamellar bodies containing a mixture of glycosphingolipids, phospholipids and ceramides, these lipids are released by the lamellar bodies into the extracellular space and strengthen the barrier function of the skin. Immune function is due to both cellular immunity (Langerhans cells or dendritic epidermal T cells) and humoral immunity. In addition, antimicrobial peptides that are produced by keratinocytes and by the cells of the immune system are involved in the process of inflammation and wound healing and are effective on a wide range of pathogens such as bacteria, fungi or viruses. By regulating the temperature and the water and electrolytes losses, the skin contributes to maintaining homeostasis. Endocrine function includes vitamin D production, and exocrine function is performed by the sebaceous and sweat glands. Through the nerve endings, the skin also has an important sensory role. The skin has a social and cosmetic role and allows the appreciation of the general state of health through the general appearance, colour or turgor. In addition, due to its permeability, the skin can be used as a means of administering drugs, with local or systemic effect.16, 17, 18, 19, 20, 21, 22, 23
FIGURE 2.
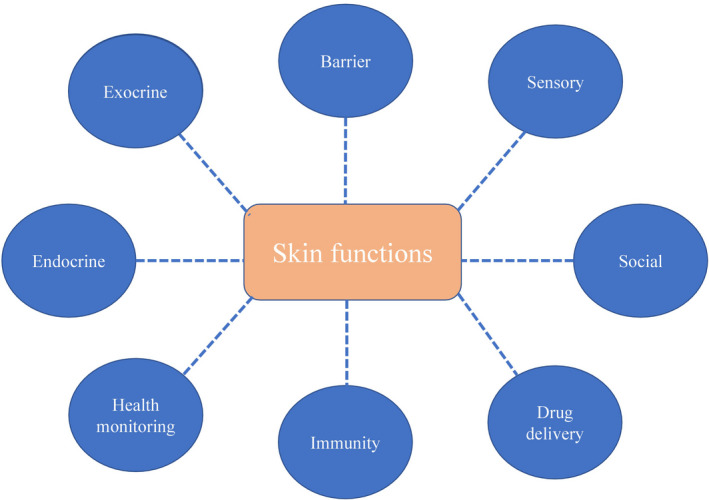
Skin functions. The barrier function is one of the most important functions of the skin, because it maintains the body's homeostasis and protects against pathogens, chemicals, radiation and mechanical damage
The barrier function of the skin is influenced by many factors. For the skin to fulfil this role, it needs to be both structurally and metabolically intact. An important role in supporting the barrier function of the skin is played by the pH value. At the level of the stratum corneum, the pH varies depending on the anatomical area, the normal values being between 4 and 5.8, while at the level of the granular layer the pH has higher values. At the level of the groin, axilla and between the toes, the pH is between 6 and 7.4. Changes in the pH value outside, the physiological limits can lead to impaired skin barrier function by altering the microbiome, lipid synthesis and enzymatic activity. In addition, the pH alteration affects the process of epidermal differentiation and desquamation. Local inflammatory pathologies (eg contact dermatitis, psoriasis, acne) altered skin barrier (eg dehydration), some systemic diseases (eg diabetes) lead to an increase in skin pH, when it approaches the value of 7. The pH of the skin can also be affected by soaps or cosmetics that are left on the skin for a long time. Bringing the pH value to a low value, in the acid spectrum, is a known therapeutic approach for wound treatment. In this process, acetic acid, ascorbic acid or hyaluronic acid can be used to control microbial colonization, influence protease activity, release oxygen, and improve epithelialization and angiogenesis. Some products dedicated to skin care have buffer capacity that allows them to adapt to local needs, beside a physiological pH value. Buffers that adjust to a specific pH may contain citrate, phosphate or glycolate, or a combination and influence skin integrity.24, 25, 26, 27 The quality of intercellular junctions also influences the barrier function of the skin. These junctions form a barrier for molecules of different sizes but also for ions, so that a poor quality affects the permeability. The junction's formation and their effectiveness depend on the presence of certain proteins such as cingulin, claudin or occludin.28, 29, 30
4. TOOLS FOR ASSESSING A PATIENT'S WOUNDS
A wound is defined as an interruption of the skin integrity, with the loss of its functions and the onset of healing mechanisms. Given the heterogeneity of causes and types of wounds, as well as patients suffering from these wounds, a standardized classification is difficult to adopt, for the medical community. Currently, clinical evaluation is the most widely used evaluation method. It is non‐invasive, fast, with a relatively optimal cost‐effectiveness ratio and can be done both in large medical units and in the outpatient department or at home. The clinical significance of a correct assessment and classification of wounds lies in the possibility of preventing complications but also of having a prognosis for morbidity, mortality and quality of life.31 In addition, it facilitates the establishment of a therapeutic plan and allows the medical team to assess whether the healing takes place within normal parameters. Given that clinical evaluation has a high degree of subjectivism, there is great variability in wound evaluation and management. This variability can be attributed to the lack of clinical experience and lack of context understanding, lack of medical staff ability to identify essential and relevant elements, lack of knowledge of treatment options and lack of ability to properly value clinical information.4 Clinical evaluation involves medical history, clinical examination and possibly the use of wound assessment charts. History taking provides information such as the mode of onset, duration, possible complications or comorbidities from the patient. The clinical examination of the wound is based on the visual description and establishes the parameters such as location, size and depth but also the evaluation of complications (tissue viability, degree of infection). Lazarus et al. (1994) describe a systematized approach when wound assessment and classification is needed and propose a number of terms and indicators. After clinical evaluation, the healthcare provider can establish three major indicators (Figure 3): wound extension, wound burden and wound severity. The degree and depth of tissue damage, reflected by wound extension, influences the therapeutic plan. The tissues that can be affected are the epidermis, dermis, subcutaneous tissue, muscles / tendon fascia or bone / viscera. Wound burden and wound severity indicate the impact that the injury has on the patient. Wound severity reflects the burden of the wound, patient‐related factors and environmental factors. Patient‐related factors refer to systemic diseases, medication and demographics that can influence wound healing. These are dynamic indicators that change over the course of the healing process or can also change in the event of environmental or patient changes, giving healthcare providers the opportunity to better monitor the patient and to adapt the treatment strategy.32
FIGURE 3.

Indicators used for wound assessment during clinical evaluation
Until now, a number of paraclinical parameters have been used to monitor and classify wounds such as microbial load, pH or temperature. In an attempt to have a more complete picture, in recent years, a number of new paraclinical examinations have been studied that can provide objective information about wound healing status using biomarkers or imaging. This information is especially useful in the context of chronic wounds. For determining these biomarkers samples such as tissue, wound fluid or the patient's blood can be used. The analytical methods used are immunohistochemistry, polymerase chain reaction (PCR), Western blot or ELISA. Following studies, a number of markers have been associated with an abnormal healing process including β‐catenin, cytokines, matrix metalloproteinase (MMP) / tissue inhibitors of metalloproteinases (TIMPs) ratio, growth factors or procalcitonin.33, 34 Imaging methods such as magnetic resonance imaging, computer tomography or ultrasound can objectively assess parameters such as size, depth, vascularity, temperature or the inflammation degree. Devices are currently being developed that can track both physical and biological parameters in real time. The developed applications and involvement of artificial intelligence increase the efficiency of these devices but also the quality of the medical act.35
5. HEALING PROCESS OF ACUTE WOUNDS
Primary wound healing, unlike secondary wound healing, involves going through all stages of healing in an orderly and well‐timed manner. The ultimate goal is to restore dermo‐epidermal integrity and function. Postnatal healing has different mechanisms and outcomes than the foetal ones.36 After the aggression, the healing process begins immediately, with the four essential phases:
Haemostasis involves vasoconstriction and platelet activation, following their contact with the extracellular matrix and damaged collagen fibres. The fibrin clot is formed, which is a temporary matrix that supports cell migration.
Inflammation with the attraction of neutrophils and monocytes from the circulation.
Cell proliferation involves re‐epithelialization, angiogenesis and granulation tissue formation, which is the second temporary matrix rich in fibroblasts and macrophages. At this stage, the fibroblasts synthesize collagen and the myofibroblasts will initiate the process of wound edges contraction.
Remodelling and maturation are processes by which the temporary matrix is replaced with the final one, organized and rich in mature collagen.
Each phase follows the previous one and for short periods there are transient overlaps. Each phase lasts from a few hours to a few months—years, depending on the wound characteristics and patient. Platelets, neutrophils, monocytes, lymphocytes and fibroblasts are cells involved in the healing process. They release cytokines, chemokines and growth factors such as vascular endothelial growth factor (VEGF), fibroblast growth factors (EGE), epidermal growth factor (EGF) or insulin‐like growth factor‐1 (IGF‐1).
In the inflammatory phase, certain molecules produced by the injured cells (hydrogen peroxide, chemokines, lipid mediators, etc.) have the role of recruiting inflammatory cells on site such as neutrophils, macrophages, dendritic cells and T cells. Neutrophils are usually the first responders and they have an important role in preventing wound infection, by releasing toxic granules, oxidative stress, phagocytosis and neutrophil extracellular traps. Studies show that the presence of macrophages at the wound site is very important, as their absence can lead to a significant neutrophil influx, and a decrease in other wound healing processes such as angiogenesis, collagen deposition and growth factors production and release.37
Reactive oxygen and nitrogen (ROS and RNS) species have a series of functions such as host defence, vasorelaxation, growth regulation and transcription. However, an imbalance in the amount of ROS/RNS produced and their elimination can lead to damaged proteins, lipids and even DNA. A recent study showed that the redox environment of the chronic wound is quite different from the acute wound. Chronic wounds appear to have significantly higher levels of TNF‐α, interleukin 8, vascular endothelial growth factor (VEGF) and lactate dehydrogenase, but the level of protein carbonylation, lipid peroxidation and tyrosine nitration was very.38
The re‐epithelization process is one of the key factors in wound healing and is dependent on keratinocytes proliferation in the basal layer, followed by their migration and differentiation to form the cornified envelope. Besides keratinocytes, proteins and lipids involved in the epithelization process must also be delivered on site.39 Peripheral blood fibrocytes were shown to stimulate cell proliferation and angiogenesis in diabetic mice, by increased synthesis of chemokines, profibrotic factors and angiogenetic factors.40
Angiogenesis is another important step in the wound healing process, by forming new blood vessels from preexisting ones, distribution throughout the wound site and the formation of the microvascular network. This neovascularization process is regulated by angiogenic cytokines in the serum (vascular endothelial growth factor, angiopoietin, fibroblast growth factor and transforming growth factor beta) and the circumferential extracellular matrix.41
There are certain situations in which the healing phases (especially the inflammatory and proliferative phase) are altered and result in chronic wounds. The causes are the inefficiency of cytokines, growth factors, proteases such as matrix metalloproteinases (‐MMPs) or cellular and extracellular elements. A poor regulation of certain stages of wound healing can lead to excessive storage of collagen resulting in defective healing and the formation of abnormal scars—hypertrophic or keloid. In addition, local or systemic processes (infections, hypoxia, diabetes) can compromise proper wound healing, with the appearance of complications and chronicity.42, 43, 44
6. HYDROGELS FOR WOUND HEALING APPLICATIONS
A hydrogel is a three‐dimensional elastic and porous network made of hydrophilic polymers, with a minimum water content of 10% (usually >90%). Hydrogels are classified according to the method of preparation, the nature of the polymers, the ionic charge or the types of bonds between the polymer chains. Depending on the monomers that compose the hydrogel, the way it is obtained and the possible additives, the hydrogels have extremely varied properties. They can respond to stimuli such as temperature, pressure, pH, ionic charge or antigens with changes in certain characteristics (e.g., gel‐soil transition) and then, when the stimulus ceases, it can return to its original state. Due to their remarkable properties, hydrogels can be included in the category of "smart" materials. In the medical field, hydrogels are used for wound treatment, drug delivery, cell therapies, tissue engineering, the manufacture of medical devices and biosensors.45, 46, 47 The use of hydrogels in the therapeutic strategy of patients suffering from wounds brings many benefits such as avoiding complications, shortening the healing time of the wound, increasing the quality of life of patients and obtaining a better therapeutic result. Hydrogels have properties that make them ideal for the treatment of deep wounds, such as non‐adhesiveness, moisture retention, gas permeability, exudate absorption, biocompatibility and are comfortable for the patient. In addition, they resemble dermal tissue, having extracellular matrix‐like structure, they allow cell migration and have the ability to induce partial tissue regeneration. A great advantage is that the intrinsic properties of hydrogels can be improved by adding active compounds, such as antibiotics, nanoparticles, stem cells and growth factors (Table 1). Hydrogels that respond to external stimuli allow controlled release of drugs or monitoring of the healing process.3, 45
TABLE 1.
Characteristics, effects and applications of hydrogels
| Types of hydrogels/applications | Basic material | Hydrogel fabrication pathways | Hydrogel effect/property | References |
|---|---|---|---|---|
| Hydrogels containing antibiotics | Polyethylene glycol (PEG) | Conjugated DNA oligonucleotides to polyethylene glycol using free radical polymerization and tetracycline | Tetracycline could inhibit bacterial growth within 48 h and induce the formation of zones of inhibition on the agar plate | 49 |
| Keratin hydrogels | Lyophilized oxidized keratin was weighed and hydrated with ciprofloxacin | Burns treated with ciprofloxacin‐ keratin hydrogels contained significantly less Pseudomonas aeruginosa and Staphylococcus aureus | 50 | |
| Polyvinyl alcohol‐gelatin (PVA) | Gentamicin and serratiopeptidase were incorporated into PVA‐gelatin hydrogel | Natural debridement by hydrating necrotic tissue with loosening and absorbing slough and exudate in wounds. It also encourages autolytic debridement | 51 | |
| Hydrogels used for the delivery of stem cells |
Adipose Extracellular Matrix (ECM), Methylcellulose (MC) |
ECM solution prepared by the dilution of lyophilized ECM in phosphate‐buffered saline solution (PBS), while MC was prepared by dispersion technique |
Accelerated wound closure, re‐epithelialization, neovascularization |
52 |
| Collagen‐ Polyethylene glycol, fibrin | Debrided skin adipose stem cells mixed with collagen and then PEG was added to the collagen, followed by the addition of fibrin |
Less wound contraction Dermal matrix deposition |
53 | |
| Pullulan–collagen | Pullulan‐collagen were added to Adipose‐derived mesenchymal stem cells suspended in growth media using a capillary force method | Accelerated wound closure, improve cell recruitment and functionality, neovascularization | 54 | |
| Pluronic F127 | Pluronic F127 powder was dissolved in PBS before encapsulation of allogeneic non‐diabetic adipose‐derived stem cells | Angiogenesis resulted from the cells in the hydrogel cell proliferation, accelerated wound closure, regeneration of granulation tissue | 55 | |
| Chitosan and gelatin | 8 ml of 2% chitosan mixed with 2 ml of 2%/4% gelatin solution. Adipose‐derived stem cells were encapsulated in the chitosan‐gelatin hydrogel | Faster cell migration at the wound site, angiogenesis, higher capillary density | 56 | |
| Hydrogels used for the delivery of bioactive agents | Mixture of polyvinyl acetate (PVA), gelatin and chitosan | 3% chitosan solution prepared in a 3% acetic acid solution; PVA was dissolved in distilled water; 5% gelatin solution dissolved in distilled water. Gel mixture was prepared in a ratio of 2:1:1 of chitosan/PVA/gelatin | Accelerated wound closure, re‐epithelization; faster transition from the inflammatory to the maturation phase, enhanced collagen deposition, myofibroblasts and vessel formation | 57 |
| Polyvinylpyrrolidone/polyethylene glycol‐dimethacrylate (PVP/PEG‐DMA) | Cyclodextrins (CD) attached to the PVP/PEG‐DMA | β‐CDs immobilized in the PVP/PEG‐DMA matrix stimulated a prolonged release of ibuprofen | 58 | |
|
Heparin Poloxamer |
Heparin–poloxamer (HP) conjugate was prepared with Ethylene dichloride/N‐hydroxysuccinimide (EDC/NHS) as coupling agents and then HP with growth factor Acid fibroblast growth factor (aFGF) hydrogels and HP‐ with growth factor Basic fibroblast growth factor (bFGF) hydrogels were prepared by lyophilizing HP powder and mixing it with aFGF / bFGF | Improved wound closure, re‐epithelialization | 59 | |
| PEG and heparin | 40% thiolated heparin and 6 kilodalton (kDa) PEG in a 1:1 ratio was dissolved in PBS | Advanced granulation tissue formation, capillary formation, and re‐epithelialization | 60 | |
| Alginate sulphate | Alginate sulphate hydrogel was made by photo‐crosslinking method and methacrylate. Then a human growth factor was added | Higher presence of collagen and of hair follicles, improved vascularization, reduced inflammation | 61 | |
| Hyaluronic Acid(HA)–MMP | Hydrogels were formed by the addition of acrylate‐ functionalized HA with bis‐cysteine containing MMP at pH 8.0–8.2 | Enhanced granulation tissue formation, angiogenesis | 62 | |
| Gelatin | Gelatin hydrogel was prepared by crosslinking of phenol with horseradish peroxidase and hydrogen peroxide | Facilitated cell infiltration into the wound area, accelerated wound healing, enhanced re‐epithelialization/neovascularization, increased collagen deposition | 63 | |
| Sodium alginate/bioglass hydrogel | 1 ml of 2% (w/v) sodium alginate solution mixed with 20 mg bioglass powders and 20 mg gluconic acid δlactone | Polarization of macrophages, upregulation of anti‐inflammatory genes, recruitment of fibroblasts and endothelial cells, improvement of the extracellular matrix | 64 | |
| Hydrogels used for skin substitution | Collagen | Collagen hydrogel was created through combination of type I collagen along with fractionated platelet‐rich plasma | Accelerated wound healing, angiogenesis, hair and sweat gland formation, regenerating a dermis‐like tissue | 67 |
| Photocrosslinkable gelatin acrylamide (GelMA) | The gelatin was modified by hydroxyphenyl propionic acid | Excellent cell viability (>90%) with increasing cell adhesion and proliferation corresponding to increases in hydrogel concentrations. Support keratinocyte growth, differentiation, and stratification into a reconstructed multilayered epidermis | 65 | |
| Pullulan‐Gelatin hydrogel | Modification of gelatin with photocrosslinkable methacrylamide groups | Promotes skin regeneration, less macrophage infiltration and increased angiogenesis; decreased inflammation | 66 | |
| Smart hydrogels stimuli‐responsive hydrogels | Aldehyde hyaluronic acid (A‐HA) / adipic acid dihydrazide graft hyaluronic acid (HA‐ADH) / sisomicin sulphate (SS) hydrogel | Evenly mixing A‐HA, HA‐ADH and SS | pH‐ and HAase‐dependent degradability that enables the release of more aminoglycosides‐SS for on‐demand and sustained anti‐infection and antioxidant activity | 68 |
| Sodium alginate/poly (N‐vinyl caprolactam) | N‐vinyl caprolactam polymerized in an aqueous sodium alginate followed by chemical and ionic crosslinking. Tannic acid incorporated hydrogels were also fabricated | Temperature‐pH dual responsive hydrogel with excellent free radical scavenging, anti‐inflammatory, antibacterial effect | 69 | |
| Alginate/polyacrylamide hydrogel matrix | Phenol red was modified with methacrylate to allow copolymerization with the hydrogel matrix | The colour of the hydrogel changes from yellow (pH 5,6 and 7) to orange (7.4 and 8), and finally to red (pH 9). This range of colour change matches the clinically meaningful pH range of chronic or infected wounds | 70 | |
| Sodium alginate/bioglass composite hydrogel | Sodium alginate (SA) microparticles containing conditioned medium (CM) of cells (SACM). Inside the SACM microparticles, poly(lactic‐co‐glycolic acid) microspheres containing pirfenidone were encapsulated | The hydrogel system sequentially delivers bioactive molecules for meeting the biologic requirements and timeline of each wound healing stage | 71 |
Abbreviations: aFGF, Acid fibroblast growth factor; A‐HA, Aldehyde hyaluronic acid; CD, Cyclodextrins; CM, Conditioned medium; ECM, Adipose Extracellular Matrix; EDC/NHS, Ethylene dichloride/N‐hydroxysuccinimide; GelMA, Photocrosslinkable gelatin acrylamide; HA, Hyaluronic Acid; HA‐ADH, Adipic acid dihydrazide graft hyaluronic acid; HP, Heparin–poloxamer; kDa, Basic fibroblast growth factor (bFGF); Kilodalton; MC, Methylcellulose; N‐vinyl caprolactam, Sodium alginate/poly; PBS, Phosphate‐buffered saline solution; PEG, Polyethylene glycol; PVA, Polyvinyl acetate; PVA, Polyvinyl alcohol‐gelatin; PVP/PEG‐DMA, Polyvinylpyrrolidone/polyethylene glycol‐dimethacrylate; SA, Sodium alginate; SS, Sisomicin sulphate.
Many research projects are now focusing on the development of new devices for wound management. There are some essential aspects that must be present in the ideal wound care system. It is very important to maintain optimal humidity at the wound site because if the surface becomes dry, it will be more difficult for the nutrients to reach the area and also the immune defences of the wound surface would become impaired, as it will take more time to reach the wound surface. The product must be non‐toxic and non‐allergenic so that it would not cause any immune response at the wound site. It is also important not to cause any harm at the wound site following the removal; it must be resistant to bacteria, should allow gaseous and water vapour exchange and it should be affordable at a large scale. Thus, one of the most used applications is the use of hydrogels for wound treatment.48
Hydrogels containing antibiotics are now used in several wound conditions, because of the capacity of the hydrogel to be non‐toxic, to have high water content, high oxygen permeability, improved biocompatibility, ease of loading and releasing drugs, structural diversity and to not cause any immune response at the wound site.49, 50, 51
Hydrogels can be used for the delivery of stem cells to the wound site. They are an attractive alternative as conveyance vehicles, as they increment the time span that stem cells live at a wound site. This property emerges from the capacity of certain hydrogels to elevate cell bonds and to engage stem cells activity by supporting the upkeep of their ordinary aggregate. These highlights are fortified by in vitro preculture of stem cells inside hydrogels, as exhibited by the presence of relocated cells in the wound site for periods longer than 11 days post‐transplantation.52, 53, 54, 55, 56
Another application of hydrogels is the delivery of bioactive agents like heparin, hyaluronic acid or ibuprofen. The properties needed by a good hydrogel are safety, antimicrobial resistance, drug loading capacity and easy drug release. It must have the capacity to maintain its properties for a long time, as some wounds would require treatment for a long period.57, 58, 59, 60, 61, 62, 63, 64
Hydrogels used for skin substitution research is still at the beginning, though some studies show promising results, Zhao et al., 2015 used a photocrosslinkable gelatin acrylamide (GelMA) that provided keratinocyte growth, differentiation and stratification into a reconstructed multilayered epidermis, as well as excellent cell viability (>90%), increased cell adhesion and proliferation of hydrogel concentrations.65 Also, they support keratinocyte growth, differentiation and stratification into a reconstructed multilayered epidermis. In another study, Nicholas et al., found in their study promising skin regeneration, less macrophage infiltration and increased angiogenesis using a Pullulan‐Gelatin hydrogel.66, 67
Smart hydrogels that respond to external stimuli can be used for monitoring wound healing, for example by incorporating a pH indicator. If the site becomes infected the pH will reach a value near 7 and the colour will change (eg from yellow to red). Another use for smart hydrogels is drug delivery according to each healing stage by incorporating active microparticles, thus sustaining the tissue regeneration process by modulating inflammation, sustaining proliferative phase and minimizing fibrosis. Moreover, depending on the wound environment, the degradation rate of hydrogels can be modified thus releasing more bioactive molecules.68, 69, 70, 71
7. CREAMS FOR WOUND HEALING APPLICATIONS
Creams are the most common types of delivery system used for emollients and moisturizers. They enable a wide variety of ingredients to be quickly and conveniently delivered to the skin.
On the base of a structural and functional definition, creams are emulsions of water in oil (oily creams) or oil in water (vanishing creams), in which the active agent is dispersed between the oil and water phases according to formula partition coefficient. The complete definition for a “cream” is suggested as an emulsion semisolid dosage form that contains >20% water and volatiles and/or <50% of hydrocarbons, waxes or polyethylene glycols as the vehicle for external application to the skin.72
By structural characteristics, creams are opaque, viscous, ranged from non‐greasy to mildly greasy texture and tend to evaporate or be absorbed when rubbed onto the skin. In comparison with ointments, creams are significantly less greasy, less viscous, less hydrating and more spreadable being used for their moistening and emollient properties.
Being a multiphase compound consisting of a lipophilic moisturizers phase and an aqueous phase, its simplest composition can immediately increase in a two‐phase system containing immiscible liquids, one of which is dispersed in the other in the form of microscopic or submicroscopic droplet, usually ranged between 1 and 100 μm.73, 74 Oil‐in‐water creams are best suited for water‐soluble drugs, while water‐in‐oil creams are best suited for lipid‐soluble drugs.72 Oil‐in‐water creams simplest form consists of aqueous phase, hydrophobic phase, and emulsifying agent. The colloidal structures, which are formed from the components, not only determine the viscoelastic properties but are also responsible for the stability of the product.75 Creams are inherently unstable and the internal phase tends to coalesce with time. A surface‐active agent tends to stabilize the emulsion by lowering the free surface energy. The degree of agitation of the two phases determines the surface area of any given internal phase volume, and consequently the stability of the system.
Creams offer many advantages over other preparations, permitting the incorporation of aqueous and oleaginous ingredients, allowing a greater release of many incorporated medicines (Table 2), and their rheological properties can be controlled. Creams are used as topical dermatological vehicles, and their influence on drug release and absorption has been studied by many authors.75, 76, 77 When a topical formulation is applied to the skin surface, it is submitted to the so‐called vehicle metamorphosis changes in composition produced primarily by evaporation of volatile components, but also by penetration of components into the skin (water, propylene glycol) and extraction of skin components (skin lipids). These changes may have a deep impact on formulation characteristics; in the specific case of emulsions, phase transitions, inversion, flocculation and coalescence can take place.78
TABLE 2.
Summary of cream types and their applications
| Type of cream | Pathology | Active substances | Activity domain | References |
|---|---|---|---|---|
| Aloe vera | Topical injuries, pathologically induced wounds, cancer treatment‐related injuries, different degree burns, skin transplant. | Flavonoid terpenoid, lectin, anthraquinone, tannin and saponin |
Antifungal, antiseptic, antiviral, antibacterial, anti‐inflammatory, antioxidant, and wound healing properties, stimulating fibroblast activity and collagen proliferation. Potential effect to increase the ratio of CD4+/CD8+ lymphocytes, CD4+ playing a crucial role as healing promotor to cellular immune response by decreasing percentage of the wound area, neutrophils infiltration, and angiogenesis |
79, 80 |
| 1% Silver Sulphadiazine Cream | Wound infections in patients with second‐ and third‐degree burns, severe burns or burns over a large area of the body | Silver nitrate, sulphonamide sodium sulphadiazine | Increase fibroblasts, macrophages and epidermal cells activities, subsequently, facilitate enzyme activity during collagen remodelling and even aids the formation of cross linkage improving in the tensile strength of the wound | 81 |
| 1% Phenytoin cream | Ulcers, epidermolysis bullosa and inflammatory conditions | 100mg Phenytoin sodium, lactose monohydrate, confectioner's sugar, talc, magnesium stearate |
Significantly increased fibroblasts generating an accelerated healing process, reducing the wound surface. Phenytoin treatment preferentially induces a Th2‐type response, depresses interferon augmentation of natural killer cell cytotoxicity in a dose‐dependent manner |
82 |
| Dexpanthenol | To treat or prevent dry, rough, scaly, itchy skin and minor skin irritations: skin burns from radiation therapy |
20.0% Dexpanthenol, 20.0% Argania spinosa kernel oil, 20.0% Polyglyceryl−3‐ polyricinoleate,30.0% Emollient, 50.0% Emulsifier and surfactants, 5.0% Antioxidants, 5.0% preservatives by weight of total composition |
Dexpanthenol is well absorbed when applied topically to the skin and rapidly converted to pantothenic acid enhancing epidermal differentiation and facilitates wound healing; it also showed activity in the prevention of biofilm formation and has anti‐inflammatory effects, moisturizer and skin barrier enhancer | 83 |
| Hirudoid, sulphonic acid mucopolysaccharide and dexamethasone | Phlebitis | Hirudoid, sulphonic acid mucopolysaccharide and dexamethasone | Improving phlebitis symptoms, shortening time of elimination of red swelling, time of pain relief at the location and time of resolution of phlebitis | 84 |
| Framycetin cream | Burns, irritation, itching and redness | Emulsifiers, waxy materials, co‐solvents, acids, preservatives, buffering agents, antioxidants, chelating agents, and humectants and water | Reduced recovery time, the absence of wound infection, the lack of redness and itching by decreasing histamine activity, up to 9 days by using it twice a day | 85 |
| Cetuximab |
Metastatic Colorectal Cancer (cancer spread beyond the colon or rectum) Squamous Cell Cancer of the Head and Neck Non‐small Cell Lung Cancer Squamous Cell Skin Cancer |
Chimeric monoclonal IgG1 antibody produced in a mammalian cell line (Sp2/0) by recombinant DNA technology | Rash induced by cancer is significantly reduced to grade 1 | 86 |
8. HYDROGELS VERSUS CREAMS
The response of hydrogels to different stimuli (temperature, pressure, pH, ionic changes, antigens, etc.) makes them very useful when we want to monitor the evolution of a wound or to counteract its degeneration by factors such as abnormal proliferation or infection. However, their high sensitivity to environmental conditions can also lead to their degradation, before fulfiling their purpose.
Hydrogels appear to be more effective in retaining moisture and the wound site than creams and they also allow unhindered cell migration, both being key factors in the rapid and efficient healing of a wound. Another fact worth mentioning is that due to their polymeric structure, they provide a better protective barrier for the wound, compared to the lipidic layer offered by creams.
As mentioned previously, studies show that hydrogels can protect stem cells and deliver them to the wound site, this finding suggests great versatility in terms of active ingredients that can be incorporated and maintained functional for extended periods of time. Maintaining the active ingredient integrity and functionality is crucial, but controlled release is also very important in the treatment of severe lesions.
Although creams do not appear to have the same versatility as hydrogels, they have their own advantages, for instance, due to their basic composition, they offer the possibility of incorporating not only water‐soluble ingredients, but also lipid‐soluble drugs.
Both hydrogels and creams have advantages and disadvantages, and their efficacy in treating different wounds is closely related to their type, depth and severity (Figure 4). For instance, Waycaster and al. (2014), have shown that patients suffering from pressure ulcer, treated with enzymatic debridement using an ointment with clostridial collagenase, presented improved healing, in terms of wound surface reduction and epithelization, compared to an autolytic debridement with a hydrogel dressing.87
FIGURE 4.
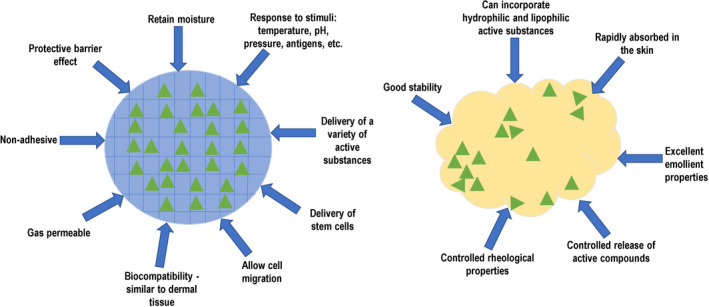
Specific properties of creams (right) and hydrogels (left) that can be both beneficial and detrimental, depending on the wound type
Another interesting study showed that a honey‐based hydrogel was not only efficient in preventing bacterial infection, but was also significantly more efficient in healing burn‐induced wounds in mice, compared to a commercially available product.88 Increased efficiency in controlling bacterial infection of burn wounds was also reported for an alginate hydrogel, with incorporated antibiotics, when compared to controls such as commercial creams, blank hydrogel, empty PWD and even intravenous antibiotic.89
Silver sulphadiazine‐based hydrogels were shown to have enhanced antimicrobial activity as opposed to commercially available creams and promoted faster wound healing (8 days instead of 12), with complete angiogenesis and re‐epithelization.90
The antimicrobial properties of silver nanoparticles are well known, but their use in wound treatment was restricted by intrinsic cytotoxic effects that reflected in skin staining, irritation and burning at wound site. Using a mix of sericin and chitosan‐capped silver nanoparticles, scientists were able to develop an efficient wound dressing, with excellent wound healing and antimicrobial properties.91
Based on all the current available data, resulted from a series of worldwide experiments, we could assume that the ideal wound dressing would have to meet many requirements, such as no toxicity, great stability, capability of wound isolation from external factors, protection for the active ingredients and controlled release at site, versatility in terms of incorporated substances. Also, the active ingredients should be selected mainly by their ability to stimulate the body's intrinsic repair capacity and antimicrobial protection. Among these nutrients are as follows: calcium, vitamin C, which promote keratinocytes differentiation; ceramide for skin fortification and natural moisturizing; glucose as an energy source for cellular proliferation and keratinocytes differentiation; vitamins such as retinol and riboflavin, that also promote cellular growth and differentiation; Vitamin D which stimulates antimicrobial protection.
9. TREATMENT OPTIONS THAT PROMOTE WOUND HEALING WHEN USED IN COMBINATION WITH HYDROGELS AND CREAMS
A study published in 2020 demonstrated the beneficial effect of carbon photon therapy, combined with a hydrogel dressing on the wound microenvironment, in a full‐thickness wound rat model. Carbon photons have been shown to produce thermal and photochemical effects, with improved microcirculation, while the hydrogel dressing had a protective effect on the wound, increased skin regeneration and minimized the drying effect of carbon light therapy.92 In another study, a hydrogel dressing combined with low‐level laser therapy showed reduction of scarring tissue, improved wound contraction and modulation of the inflammation process.93
A recent approach for wound healing used a microcurrent device, composed of silver and zinc nanoparticles and a medical cotton cushion. The mixture of silver and zinc nanoparticles was then sprayed on one side of the cotton cushion, while the other side was covered with a thin film, in order to retain moisture and allow gas exchange. The results revealed reduced cytokine expression and an increase in the levels of EGF and VEGF factors that promote angiogenesis and growth of keratinocytes.94 Because of their moisture‐retaining properties and gas permeability, hydrogels could be used in association with the microcurrent technology, in order to improve their beneficial effects. To improve wound healing by modulating local microenvironment researchers developed a Chitosan‐Vaseline® gauze combined with a flexible electrical stimulation device. Results showed that high voltage monophasic pulsed current increased vascular endothelial growth factor levels, promoted cell migration and proliferation and decreased scar tissue formation.95
Another promising alternative is the on‐site production of CXCL12 (C‐X‐C Motif Chemokine Ligand 12) by lactic acid bacteria, which was shown to increase dermal cells and macrophages proliferation and higher values of TGF‐β, which manifested in a higher wound closure rate.96 These modified bacterial cells could be embedded in custom designed creams or hydrogels for the optimum delivery and controlled release of CXCL12 on site. In another study, a biologic gel containing Synechococcus elongatus, a bacteria that can provide oxygen via photosynthesis to ischaemic tissue, was used in a rat model with peripheral arterial disease to treat skin wounds. The results showed that healing time was reduced compared to control groups and that none had positive blood culture, indicating that the bacteria did not passed the biological barrier.97
The transduction of four transcription factors involved in cell differentiation has been proven to trick mesenchymal cells into becoming epithelial cells, thus accelerating the wound healing process. In order to assess the efficacy of the DGTM factors (DNP63A, GRHL2, TFAP2A and MYC), in vivo researchers used an adenovirus (AAV) and the green fluorescent protein (GFP). To further improve the clinical relevance of the designed therapy, they enhanced the AAV‐GFP complex delivery by incorporating it into a collagen gel. Results showed that by using the collagen gel both GFP expression and AAV copy number in the ulcer tissue were increased and the overall efficacy of the AAV system was improved.98 An adult kidney 2 human (HK2) cell line, with epithelial morphology, was used to obtain nephron progenitor cells using 15 different transcriptional factors.99 Besides integration, transcription factors can also be transported via non‐integrating methods, such as chemical or physical ones.100
The new trend in wound therapy is the development of all‐in‐one patches, with the ability to monitor wound status and also at‐site release of the optimal amount of active compounds. Pang et al, developed a flexible electronics‐integrated wound dressing with a double layer. One layer contains the electronic part encapsulated in polydimethylsiloxane with a temperature sensor and UV light emitting diodes, while the other layer is a UV‐responsive antibacterial hydrogel. The purpose of the “intelligent patch” is the detection of infection in its early stages and the controlled release of antibiotics on‐site, in order to prevent complications.101
Another interesting device for wound monitoring and controlled drug release was developed using a silk fibroin microneedle structure, with a temperature‐responsive hydrogel made from N‐isopropylacrylamide (NIPAM) and opal photonic crystals. This new intelligent dressing allowed the monitoring of multiple inflammatory factors and wound motion (such as stretching) and the controlled release of drugs, which lead to a faster recovery rate in diabetic mice.102
Skin‐wound therapy can also be revolutionized by next‐generation technology such as four‐dimensional (4D) printing. Evidence of developing trachea mimetic tissue was already offered by a recent study. To obtain a tissue similar to that of the trachea, the base layer of hydrogel sheet was made of human turbinate‐derived stem cells (hTBSCs) and the patterned layer contained human chondrocytes for the hyaline cartilage ring. The 4D‐bioprinted hydrogels that were implanted into a rabbit with damaged trachea has excellent shape‐morphing controllability, reliability and biocompatibility. Also, the implantation of the obtained tissue in the host trachea determined the formation of both epithelium and cartilage.103
10. CONCLUSIONS
Both creams and hydrogels have proven efficient in preventing wound infection and stimulating cell proliferation, growth and differentiation, but their efficacy is clearly conditioned by the type, depth, severity of the wound and the patient's health status. Hydrogels appear to be more suited for the development of the ideal wound dressing, due to their many beneficial properties and versatility. The development of dressings that could suit all types of wounds is still very difficult to imagine, and the secret of therapeutic success of patients with wounds lies in the knowledge of medical staff about the pathophysiology of wounds and treatment options. Development of new technologies and increased access to them in medical practice bring new treatment resources for patients but their successful use requires a good knowledge of therapeutic indications. Researchers in the field can develop intelligent devices that allow both wound monitoring and the delivery of biologically active substances. In addition, due to technological advancement, smart dressings can combine physical therapies that use the beneficial effects of electrical simulation or photons. These devices have the potential to increase patient compliance and improve therapeutic outcome if used at the right time to the right patient.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
D.S. wrote the chapter on skin functions and contributed to the chapter about hydrogels. C.T. wrote the chapter on skin anatomy. M.A. helped to write the chapter on hydrogels. R.A. wrote the chapter on creams. N.B.M did the documentation and editing of the hydrogels chapter. A.L.M. made the comparison between creams and hydrogels. D.S. designed, corrected and edited the manuscript.
ACKNOWLEDGMENTS
This work was supported by the project INTELBIOMED, Ctr. No. 925/03.07.2019, code P_40_197, SMIS2014 105631 and co‐financed by DDS Diagnostic SRL.
Stan D, Tanase C, Avram M, et al. Wound healing applications of creams and “smart” hydrogels. Exp Dermatol. 2021;30:1218–1232. 10.1111/exd.14396
REFERENCES
- 1.Nussbaum SR, Carter MJ, Fife CE, et al. An economic evaluation of the impact, cost, and medicare policy implications of chronic nonhealing wounds. Value Health. 2018;21(1):27‐32. [DOI] [PubMed] [Google Scholar]
- 2.Lindholm C, Searle R. Wound management for the 21st century: combining effectiveness and efficiency. Int Wound J. 2016;13(Suppl 2):5‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Francesko A, Petkova P, Tzanov T. Hydrogel dressings for advanced wound management. Curr Med Chem. 2018;25(41):5782‐5797. [DOI] [PubMed] [Google Scholar]
- 4.Logan G. Clinical judgment and decision‐making in wound assessment and management: is experience enough? British Journal of Community Nursing. 2015;20(Sup3):S21‐S28. [DOI] [PubMed] [Google Scholar]
- 5.Bruce MC. Human Embryology and Developmental Biology. St. Louis: Elsevier; 2018:156p. [Google Scholar]
- 6.Drake RL, Vogl W, Mitchell A. Gray's anatomy for students. Philadelphia: Elsevier/Churchill Livingstone; 2009:23p. [Google Scholar]
- 7.McGrath JA, Uitto J. Structure and Function of the Skin. Rook's Textbook of Dermatology. John Wiley & Sons. 2016;3p. [Google Scholar]
- 8.Cumberbatch M, Dearman RJ, Griffiths CE, Kimber I. Epidermal Langerhans cell migration and sensitisation to chemical allergens. APMIS. 2003;111(7–8):797‐804. [DOI] [PubMed] [Google Scholar]
- 9.Randall RW, Marty OV. Structure and function of the epidermal barrier. Am. J. Infect. Control. 2006;34(10):98‐110. [Google Scholar]
- 10.Hom DB, Patricia H, Arun G, Craig F. Essential tissue healing of the face and neck. PMPH USA. 2009;10‐25p. [Google Scholar]
- 11.Steinert PM. Structure, function, and dynamics of keratin intermediate filaments. J Invest Dermatol. 1993;100(6):729‐734. [DOI] [PubMed] [Google Scholar]
- 12.Stecco C, Pirri C, Fede C, et al. Dermatome and fasciatome. Clin Anat. 2019;32(7):896‐902. [DOI] [PubMed] [Google Scholar]
- 13.Turcan I, Jonkman MF. Blistering disease: insight from the hemidesmosome and other components of the dermal‐epidermal junction. Cell Tissue Res. 2015;360(3):545‐569. [DOI] [PubMed] [Google Scholar]
- 14.Turner CT, Hiroyasu S, Granville DJ. Granzyme B as a therapeutic target for wound healing. Expert Opin Ther Targets. 2019;23(9):745‐754. [DOI] [PubMed] [Google Scholar]
- 15.Driskell RR, Lichtenberger BM, Hoste E, et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature. 2013;504(7479):277‐281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iheanacho F, Vellipuram AR. Mechanoreceptors. In: StatPearls. Treasure Island (FL): StatPearls Publishing, 2020. [PubMed] [Google Scholar]
- 17.Dąbrowska AK, Rotaru GM, Derler S, et al. Materials used to simulate physical properties of human skin. Skin Res Technol. 2016;22(1):3‐14. [DOI] [PubMed] [Google Scholar]
- 18.Dąbrowska AK, Spano F, Derler S, Adlhart C, Spencer ND, Rossi RM. The relationship between skin function, barrier properties, and body‐dependent factors. Skin Res Technol. 2018;24(2):165‐174. [DOI] [PubMed] [Google Scholar]
- 19.Feingold KR. Lamellar bodies: the key to cutaneous barrier function. J Invest Dermatol. 2012;132(8):1951‐1953. [DOI] [PubMed] [Google Scholar]
- 20.Prausnitz MR. Engineering microneedle patches for vaccination and drug delivery to skin. Annu Rev Chem Biomol Eng. 2017;8:177‐200. [DOI] [PubMed] [Google Scholar]
- 21.Yousef H, Alhajj M, Sharma S. Anatomy, skin (integument), epidermis. StatPearls. Treasure Island (FL): StatPearls Publishing. 2020. [PubMed] [Google Scholar]
- 22.Kim HS, Sun X, Lee JH, Kim HW, Fu X, Leong KW. Advanced drug delivery systems and artificial skin grafts for skin wound healing. Adv Drug Deliv Rev. 2019;146:209‐239. [DOI] [PubMed] [Google Scholar]
- 23.Hilchie AL, Wuerth K, Hancock RE. Immune modulation by multifaceted cationic host defense (antimicrobial) peptides. Nat Chem Biol. 2013;9(12):761‐768. [DOI] [PubMed] [Google Scholar]
- 24.Proksch E. pH in nature, humans and skin. J Dermatol. 2018;45(9):1044‐1052. [DOI] [PubMed] [Google Scholar]
- 25.Schmid‐Wendtner MH, Korting HC. The pH of the skin surface and its impact on the barrier function. Skin Pharmacol Physiol. 2006;19(6):296‐302. [DOI] [PubMed] [Google Scholar]
- 26.Kleesz P, Darlenski R, Fluhr JW. Full‐body skin mapping for six biophysical parameters: baseline values at 16 anatomical sites in 125 human subjects. Skin Pharmacol Physiol. 2012;25(1):25‐33. [DOI] [PubMed] [Google Scholar]
- 27.Hachem JP, Roelandt T, Schürer N, et al. Acute acidification of stratum corneum membrane domains using polyhydroxyl acids improves lipid processing and inhibits degradation of corneodesmosomes. J Invest Dermatol. 2010;130(2):500‐510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bäsler K, Bergmann S, Heisig M, Naegel A, Zorn‐Kruppa M, Brandner JM. The role of tight junctions in skin barrier function and dermal absorption. J Control Release. 2016;242:105‐118. [DOI] [PubMed] [Google Scholar]
- 29.Kirschner N, Houdek P, Fromm M, Moll I, Brandner JM. Tight junctions form a barrier in human epidermis. Eur J Cell Biol. 2010;89(11):839‐842. [DOI] [PubMed] [Google Scholar]
- 30.Mertens AE, Rygiel TP, Olivo C, Van der Kammen R, Collard JG. The Rac activator Tiam1 controls tight junction biogenesis in keratinocytes through binding to and activation of the Par polarity complex. J Cell Biol. 2005;170(7):1029‐1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mioton LM, Jordan SW, Hanwright PJ, Bilimoria KY, Kim JY. The Relationship between preoperative wound classification and postoperative infection: a multi‐institutional analysis of 15,289 patients. Arch Plast Surg. 2013;40(5):522‐529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lazarus GS, Cooper DM, Knighton DR, Percoraro RE, Rodeheaver G, Robson MC. Definitions and guidelines for assessment of wounds and evaluation of healing. Wound Repair Regen. 1994;2(3):165‐170. [DOI] [PubMed] [Google Scholar]
- 33.Lindley LE, Stojadinovic O, Pastar I, Tomic‐Canic M. Biology and biomarkers for wound healing. Plast Reconstr Surg. 2016;138(3 Suppl):18S‐28S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel S, Maheshwari A, Chandra A. Biomarkers for wound healing and their evaluation. J Wound Care. 2016;25(1):46‐55. [DOI] [PubMed] [Google Scholar]
- 35.Li S, Mohamedi AH, Senkowsky J, Nair A, Tang L. Imaging in chronic wound diagnostics. Adv Wound Care (New Rochelle). 2020;9(5):245‐263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muntean A, Stoica I, Enescu DM. Scarless wound healing – a literature review. Ro J Pediat. 2019;68(3):160‐165. [Google Scholar]
- 37.Rodrigues M, Kosaric N, Bonham CA, Gurtner GC. Wound healing: a cellular perspective. Physiol Rev. 2019;99(1):665‐706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bodnár E, Bakondi E, Kovács K, et al. Redox profiling reveals clear differences between molecular patterns of wound fluids from acute and chronic wounds. Oxid Med Cell Longev. 2018;2018:5286785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pastar I, Stojadinovic O, Yin NC, et al. Epithelialization in wound healing: a comprehensive review. Adv Wound Care (New Rochelle). 2014;3(7):445‐464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kao HK, Chen B, Murphy GF, Li Q, Orgill DP, Guo L. Peripheral blood fibrocytes: enhancement of wound healing by cell proliferation, re‐epithelialization, contraction, and angiogenesis. Ann Surg. 2011;254(6):1066‐1074. [DOI] [PubMed] [Google Scholar]
- 41.Honnegowda TM, Pramod K, Echalasara GPU, Sudesh K, Udaya K, Pragna R. Role of angiogenesis and angiogenic factors in acute and chronic wound healing. Plast Aesthet Res. 2015;2:243‐249. [Google Scholar]
- 42.Stuart E, David JL. Basic science of wound healing. Surgery (Oxford). 2008;26(2):31. [Google Scholar]
- 43.Singh N, Bhattacharyya D. Proteases in Wound Healing and Immunity. In: Chakraborti S, Chakraborti T, Dhalla N, eds. Proteases in Human Diseases. Springer: Singapore; 2017:147‐170p. [Google Scholar]
- 44.Tottoli EM, Dorati R, Genta I, Chiesa E, Pisani S, Conti B. Skin wound healing process and new emerging technologies for skin wound care and regeneration. Pharmaceutics. 2020;12(8):735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Calo E, Ballamy L, Khutoryanskiy VV. Hydrogels in wound management. In Hydrogels: Design, Synthesis and Application. Drug Delivery and Regenerative Medicine; 2018. London: CRC Press. [Google Scholar]
- 46.Chirani N, Gritsch L, Motta FL, Fare S. History and applications of hydrogels. J Biomed Sci. 2015;4:1‐23. [Google Scholar]
- 47.Morteza B, Naimeh M, Mehdi M. An Introduction to Hydrogels and Some Recent Applications, Emerging Concepts in Analysis and Applications of Hydrogels. IntechOpen. 2016. Available from:https://www.intechopen.com/books/emerging‐concepts‐in‐analysis‐and‐applications‐of‐hydrogels/an‐introduction‐to‐hydrogels‐and‐some‐recent‐applications. Accessed January 2021. [Google Scholar]
- 48.Tavakoli S, Klar A. Advanced hydrogels as wound dressings. Biomolecules. 2020;10(8):1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang X, Soontornworajit B, Zhang Z, Chen N, Wang Y. Enhanced loading and controlled release of antibiotics using nucleic acids as an antibiotic‐binding effector in hydrogels. Biomacromol. 2012;13(7):2202‐2210. [DOI] [PubMed] [Google Scholar]
- 50.Roy D, Tomblyn S, Isaac K, et al. Ciprofloxacin‐loaded keratin hydrogels reduce infection and support healing in a porcine partial‐thickness thermal burn. Wound Repair Regen. 2016;24(4):657‐668. [DOI] [PubMed] [Google Scholar]
- 51.Singh D, Singh M. Development of antibiotic and debriding enzyme‐loaded PLGA microspheres entrapped in PVA‐gelatin hydrogel for complete wound management. Artif Cells Blood Substit Biotechnol. 2012;40(5):345‐353. [DOI] [PubMed] [Google Scholar]
- 52.Kim Y, Tabata Y. Recruitment of mesenchymal stem cells and macrophages by dual release of stromal cell‐derived factor‐1 and a macrophage recruitment agent enhances wound closure. J Biomed Mater Res Part A. 2016;104(4):942‐956. [DOI] [PubMed] [Google Scholar]
- 53.Natesan S, Zamora D, Wrice N, Baer D, Christy R. Bilayer hydrogel with autologous stem cells derived from debrided human burn skin for improved skin regeneration. J Burn Care Res. 2013;34(1):18‐30. [DOI] [PubMed] [Google Scholar]
- 54.Kosaraju R, Rennert RC, Maan ZN, et al. Adipose‐derived stem cell‐seeded hydrogels increase endogenous progenitor cell recruitment and neovascularization in wounds. Tissue Eng Part A. 2016;22(3–4):295‐305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaisang L, Siyu W, Lijun F, Daoyan P, Xian C, Jie S. Adipose‐derived stem cells seeded in Pluronic F‐127 hydrogel promotes diabetic wound healing. J Surg Res. 2017;217:63‐74. [DOI] [PubMed] [Google Scholar]
- 56.Cheng N, Lin W, Ling T, Young T. Sustained release of adipose‐derived stem cells by thermosensitive chitosan/gelatin hydrogel for therapeutic angiogenesis. Acta Biomater. 2017;51:258‐267. [DOI] [PubMed] [Google Scholar]
- 57.Shamloo A, Sarmadi M, Aghababaie Z, Vossoughi M. Accelerated full‐thickness wound healing via sustained bFGF delivery based on a PVA/chitosan/gelatin hydrogel incorporating PCL microspheres. Int J Pharm. 2018;537(1–2):278‐289. [DOI] [PubMed] [Google Scholar]
- 58.Nielsen A, Madsen F, Larsen K. Cyclodextrin modified hydrogels of PVP/PEG for sustained drug release. Drug Deliv. 2009;16(2):92‐101. [DOI] [PubMed] [Google Scholar]
- 59.Wu J, Zhu J, He C, et al. Comparative study of heparin‐poloxamer hydrogel modified bFGF and aFGF for in vivo wound healing efficiency. ACS Appl Mater Interfaces. 2016;8(29):18710‐18721. [DOI] [PubMed] [Google Scholar]
- 60.Goh M, Hwang Y, Tae G. Epidermal growth factor loaded heparin‐based hydrogel sheet for skin wound healing. Carbohydr Polym. 2016;147:251‐260. [DOI] [PubMed] [Google Scholar]
- 61.Babavalian H, Tebyanian H, Latifi A, Shokrgozar M, Bonakdar S, Shakeri F. The effect of synthetic alginate sulfate hydrogels with recombinant PDGF‐BB on Wound healing. Bratislava Med J. 2018;119(06):391‐396. [DOI] [PubMed] [Google Scholar]
- 62.Tokatlian T, Cam C, Segura T. Porous hyaluronic acid hydrogels for localized nonviral DNA delivery in a diabetic wound healing model. Adv Healthc Mater. 2015;4(7):1084‐1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yoon D, Lee Y, Ryu H, et al. Cell recruiting chemokine‐loaded sprayable gelatin hydrogel dressings for diabetic wound healing. Acta Biomater. 2016;38:59‐68. [DOI] [PubMed] [Google Scholar]
- 64.Zhu Y, Ma Z, Kong L, He Y, Chan H, Li H. Modulation of macrophages by bioactive glass/sodium alginate hydrogel is crucial in skin regeneration enhancement. Biomaterials. 2020;256:120216. [DOI] [PubMed] [Google Scholar]
- 65.Zhao X, Lang Q, Yildirimer L, et al. Photocrosslinkable gelatin hydrogel for epidermal tissue engineering. Adv Healthc Mater. 2015;5(1):108‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nicholas M, Jeschke M, Amini‐Nik S. Cellularized bilayer pullulan‐gelatin hydrogel for skin regeneration. Tissue Eng Part A. 2016;22(9–10):754‐764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Houdek M, Wyles C, Stalboerger P, Terzic A, Behfar A, Moran S. Collagen and fractionated platelet‐rich plasma scaffold for dermal regeneration. Plast Reconstr Surg. 2016;137(5):1498‐1506. [DOI] [PubMed] [Google Scholar]
- 68.Guan S, Li Y, Cheng C, et al. Manufacture of pH‐ and HAase‐responsive hydrogels with on‐demand and continuous antibacterial activity for full‐thickness wound healing. Int J Biol Macromol. 2020;164:2418‐2431. [DOI] [PubMed] [Google Scholar]
- 69.Preman NK, Sindhu Priya ES, Prabhu A, et al. Bioresponsive supramolecular hydrogels for hemostasis, infection control and accelerated dermal wound healing. J Mater Chem B. 2020;8(37):8585‐8598. [DOI] [PubMed] [Google Scholar]
- 70.Liu L, Li X, Nagao M, Elias AL, Narain R, Chung HJ. A pH‐Indicating Colorimetric Tough Hydrogel Patch towards Applications in a Substrate for Smart Wound Dressings. Polymers (Basel). 2017;9(11):558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ma Z, Song W, He Y, Li H. Multilayer injectable hydrogel system sequentially delivers bioactive substances for each wound healing stage. ACS Appl Mater Interfaces. 2020;12(26):29787‐29806. [DOI] [PubMed] [Google Scholar]
- 72.Mayba JN, Gooderham MJ. Review of atopic dermatitis and topical therapies. J Cutan Med Surg. 2017;21(3):227‐236. [DOI] [PubMed] [Google Scholar]
- 73.Lodén M. Role of topical emollients and moisturizers in the treatment of dry skin barrier disorders. Am J Clin Dermatol. 2003;4(11):771‐788. [DOI] [PubMed] [Google Scholar]
- 74.Yoshida Y, Hashimoto K, Saeki H, et al. Efficacy of a moisturizer for pruritus accompanied with asteatosis in dialysis patients: an open‐label, randomized, exploratory study. Kidney Med. 2019;1(4):191‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Okamoto T, Tomomasa S, Nakajima H. Preparation and thermal properties of fatty alcohol/surfactant/oil/water nanoemulsions and their cosmetic applications. J Oleo Sci. 2016;65(1):27‐36. [DOI] [PubMed] [Google Scholar]
- 76.Sepulveda E, Kildsig DO, Ghaly ES. Relationship between internal phase volume and emulsion stability: the cetyl alcohol/stearyl alcohol system. Pharm Dev Technol. 2003;8(3):263‐275. [DOI] [PubMed] [Google Scholar]
- 77.Farooq A, Shafaghat H, Jae J, Jung SC, Park YK. Enhanced stability of bio‐oil and diesel fuel emulsion using Span 80 and Tween 60 emulsifiers. J Environ Manag. 2019;231:694‐700. [DOI] [PubMed] [Google Scholar]
- 78.Fantini A, Demurtas A, Nicoli S, Padula C, Pescina S, Santi P. In vitro skin retention of crisaborole after topical application. Pharmaceutics. 2020;12(6):491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rahman S, Carter P, Bhattarai N. Aloe vera for tissue engineering applications. J Funct Biomater. 2017;8(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Prakoso YA, Kurniasih. The effects of Aloe vera cream on the expression of CD4+ and CD8+ lymphocytes in skin wound healing. J Trop Med. 2018;2018:6218303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tarameshloo M, Norouzian M, Zarein‐Dolab S, Dadpay M, Gazor R. A comparative study of the effects of topical application of Aloe vera, thyroid hormone and silver sulfadiazine on skin wounds in Wistar rats. Lab Anim Res. 2012;28(1):17‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Takzaree N, Hadjiakhondi A, Hassanzadeh G, Rouini MR, Manayi A, Zolbin MM. Transforming growth factor‐β (TGF‐β) activation in cutaneous wounds after topical application of aloe vera gel. Can J Physiol Pharmacol. 2016;94(12):1285‐1290. [DOI] [PubMed] [Google Scholar]
- 83.Gorski J, Proksch E, Baron JM, Schmid D, Zhang L. Dexpanthenol in wound healing after medical and cosmetic interventions (postprocedure wound healing). Pharmaceuticals (Basel). 2020;13(7):138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zheng GH, Yang L, Chen HY, Chu JF, Mei L. Aloe vera for prevention and treatment of infusion phlebitis. Cochrane Database Syst Rev. 2014;2014(6):CD009162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hekmatpou D, Mehrabi F, Rahzani K, Aminiyan A. The effect of aloe vera clinical trials on prevention and healing of skin wound: a systematic review. Iran J Med Sci. 2019;44(1):1‐9. [PMC free article] [PubMed] [Google Scholar]
- 86.Gürbüz M, Akkuş E, Utkan G. Topical aloe vera for the treatment of cetuximab‐related acneiform rash in colorectal cancer: a case report. J Oncol Pharm Pract. 2021;27(2):480‐484. 10.1177/1078155220937751. [DOI] [PubMed] [Google Scholar]
- 87.Curtis R. Waycaster, Adrienne M Gilligan, Catherine T Milne, Pressure ulcer treatment in a long‐term care setting: wound bed healing with clostridial collagenase ointment versus hydrogel dressing. Chronic Wound Care Manag Res. 2014;1:49‐56. [Google Scholar]
- 88.El‐Kased RF, Amer RI, Attia D, Elmazar MM. Honey‐based hydrogel: In vitro and comparative In vivo evaluation for burn wound healing. Sci Rep. 2017;7(1):9692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nuutila K, Grolman J, Yang L, et al. Immediate treatment of burn wounds with high concentrations of topical antibiotics in an alginate hydrogel using a platform wound device. Adv Wound Care (New Rochelle). 2020;9(2):48‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pandey A, Momin M, Chando A. Silver sulfadiazine loaded breathable hydrogel sponge for wound healing. Drug Metab Pers Ther. 2020;35(3). 10.1515/dmpt-2020-0124. [DOI] [PubMed] [Google Scholar]
- 91.Verma J, Kanoujia J, Parashar P, Tripathi CB, Saraf SA. Wound healing applications of sericin/chitosan‐capped silver nanoparticles incorporated hydrogel. Drug Deliv Transl Res. 2017;7(1):77‐88. [DOI] [PubMed] [Google Scholar]
- 92.Yang F, Zhou X, Chen S, et al. Combined carbon photon and hydrogel therapy mediates the synergistic repair of full‐thickness skin wounds. J Int Med Res. 2020;48(8):300060520935326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Aragão‐Neto AC, Soares PA, Lima‐Ribeiro MH, Carvalho EJ, Correia MT, Carneiro‐da‐Cunha MG. Combined therapy using low level laser and chitosan‐policaju hydrogel for wound healing. Int J Biol Macromol. 2017;95:268‐272. [DOI] [PubMed] [Google Scholar]
- 94.Yu C, Xu Z‐X, Hao Y‐H, et al. A novel microcurrent dressing for wound healing in a rat skin defect model. Milit Med Res. 2019;6(1):22. 10.1186/s40779-019-0213-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang XF, Li ML, Fang QQ, et al. Flexible electrical stimulation device with Chitosan‐Vaseline® dressing accelerates wound healing in diabetes. Bioact Mater. 2020;6(1):230‐243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vågesjö E, Öhnstedt E, Mortier A, et al. Accelerated wound healing in mice by on‐site production and delivery of CXCL12 by transformed lactic acid bacteria. Proc Natl Acad Sci USA. 2018;115(8):1895‐1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yuanjia Z, Jinsuh J, Shreya A, et al. A novel photon‐powered biologic gel for enhanced wound healing in a peripheral arterial disease model. Circulation. 2020;142:A13909. [Google Scholar]
- 98.Kurita M, Araoka T, Hishida T, et al. In vivo reprogramming of wound‐resident cells generates skin epithelial tissue. Nature. 2018;561(7722):243‐247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hendry CE, Vanslambrouck JM, Ineson J, et al. Direct transcriptional reprogramming of adult cells to embryonic nephron progenitors. J Am Soc Nephrol. 2013;24(9):1424‐1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Begum S. Engineering renal epithelial cells: programming and directed differentiation towards glomerular podocyte's progenitor and mature podocyte. Am J Transl Res. 2019;11(2):1102‐1115. [PMC free article] [PubMed] [Google Scholar]
- 101.Pang Q, Lou D, Li S, et al. Smart flexible electronics‐integrated wound dressing for real‐time monitoring and on‐demand treatment of infected wounds. Adv Sci (Weinh). 2020;7(6):1902673. 10.1002/advs.201902673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gao BB, Guo MZ, Lyu K, Chu TS, He BF. Intelligent silk fibroin based microneedle dressing (i‐SMD). Adv Funct Mater. 2021;31:2006839. [Google Scholar]
- 103.Kim SH, Seo YB, Yeon YK, et al. 4D‐bioprinted silk hydrogels for tissue engineering. Biomaterials. 2020;260:120281. [DOI] [PubMed] [Google Scholar]


